Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise SOLVED EXAMPLES|20 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE-1|4 VideosCHEMICAL EQUILIBRIUM
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosCHEMICAL BONDING-I & II
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|98 VideosCHEMICAL KINETICS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|52 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-CHEMICAL EQUILIBRIUM-Illustration
- A vessel at 1000 K contains carbon dioxide with a pressure of 0.5 atm....
Text Solution
|
- When S in the form of S(8) is heated at 900 K, the initial pressure of...
Text Solution
|
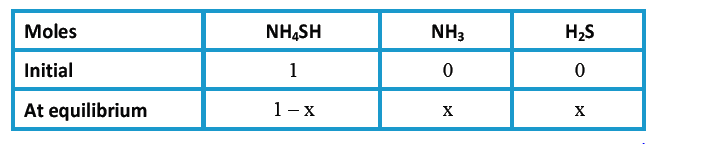
- Solid NH(4)HS(s) (ammonium hydrogen sulphate) dissociates to give NH(3...
Text Solution
|
- The K(c) for A(2(g))+B(2(g))hArr2AB((g)) at 100^(@)C is 50. If one lit...
Text Solution
|
- In the reaction, H(2)(g)+I(2)(g)hArr2HI(g) The concentration of H(...
Text Solution
|
- The value of K(c) for the reaction: H(2)(g)+I(2)(g) hArr 2HI (g) is 48...
Text Solution
|
- For the reaction, N(2)(g)+3H(2)(g) hArr 2NH(3)(g) the partial pres...
Text Solution
|
- At 1000K, the pressure of iodine gas is found to be 0.112 atm due to p...
Text Solution
|
- Calculate the precentage dissociation of H(2)S(g) if 0.1 mol of H(2)S ...
Text Solution
|
- For the reaction CO(2)(g)+H(2)(g) hArr CO(g)+H(2)O(g) K is 0.63 at...
Text Solution
|
- What would be the effect of increasing the volume of each of the follo...
Text Solution
|
- What happens when an inert gas is added to i. PCl(5)(g) hArr PCl(3)(...
Text Solution
|
- For the dissociation of gaseous HI, 2HI(g) hArr H(2)(g)+I(2)(g). If 2 ...
Text Solution
|
- The equilibrium constant for the reaction H(2)(g)+S(s) hArr H(2)S(g)...
Text Solution
|
- When PCl(5) is heated, it dissociates into PCl(3) and Cl(2). The vapou...
Text Solution
|
- The vapour density of N(2)O(4) at a certain temperature is 30. Calcula...
Text Solution
|
- K(p) for the reaction PCl(5)(g)ghArrPCl(3)(g)+Cl(2)(g) at 250^(@)C...
Text Solution
|
- At a given temperature and a total pressure of 1.0 atm for the homogen...
Text Solution
|
- One mole of N(2) and 3 moles of PCl(5) are placed in a 100L vessel hea...
Text Solution
|
- At temperature ,T, a compound AB2(g) dissociates according to the rea...
Text Solution
|