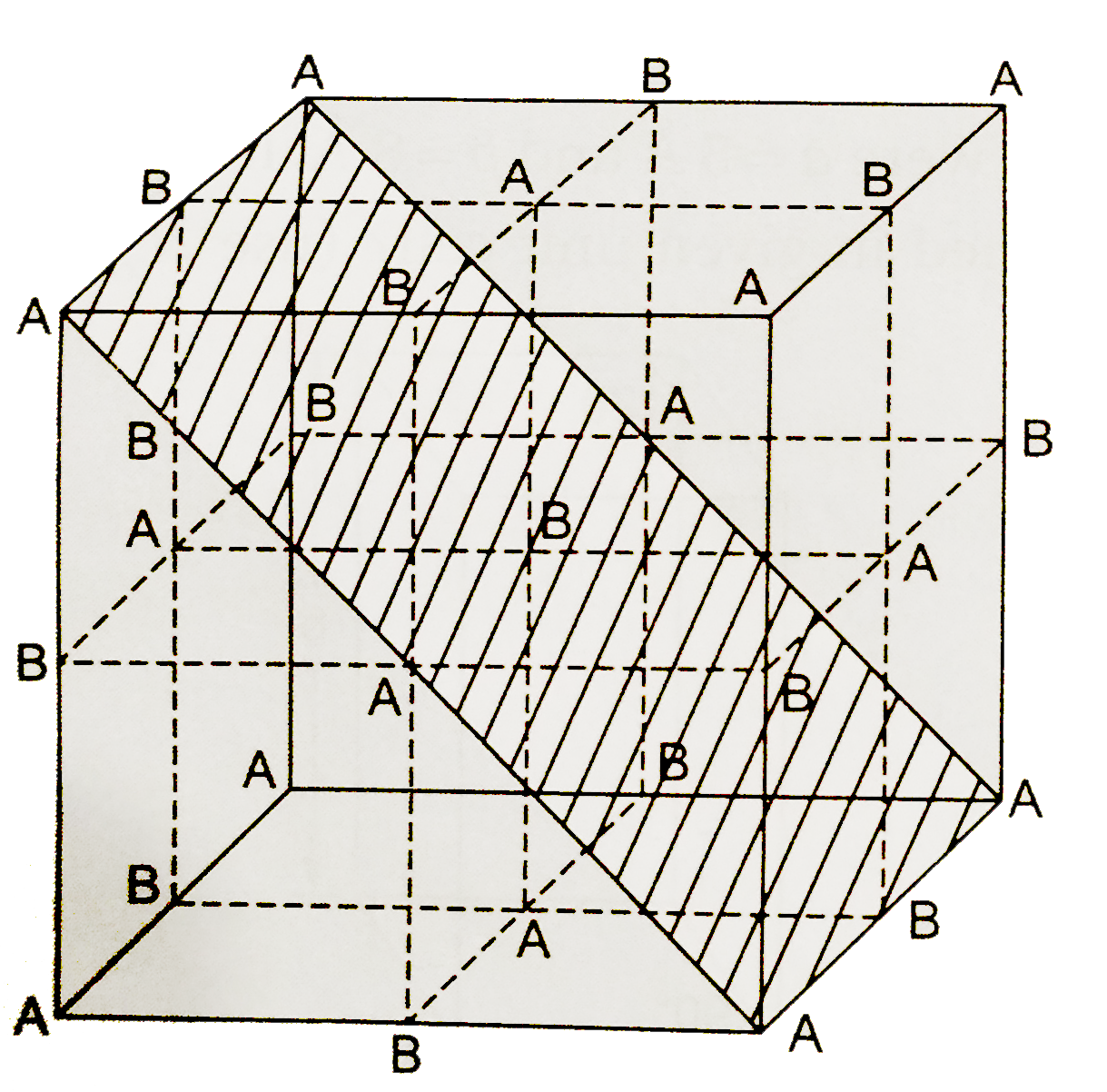
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
THE SOLID STATE
VMC MODULES ENGLISH|Exercise EXAMPLE|20 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise EXERCISE - A|10 VideosTHE SOLID STATE
VMC MODULES ENGLISH|Exercise IMPECCABLE|49 VideosSURFACE CHEMISTRY
VMC MODULES ENGLISH|Exercise PRACTICE EXERCISE|9 VideosTHEORY OF SOLUTIONS
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|31 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-THE SOLID STATE-ILLUSTRATIONS
- A solid has a cubic structure in which X atoms are located at the corn...
Text Solution
|
- A crystal is made of particles A and B . From fcc packing and B...
Text Solution
|
- In a solid having rock salt structure, if all tge atoms touching one b...
Text Solution
|
- In a close packed structure of mixed oxides, the lattice is composed ...
Text Solution
|
- An ionic solid A^(o+)B^(Θ) crystallizes as an bcc structure. The dist...
Text Solution
|
- Potassium crystallizes in a body centered cubic lattice. What is the a...
Text Solution
|
- Calculate the packing fraction for the Ca unit cell, given that Ca cry...
Text Solution
|
- A compound formed by elements A and B crystallizes in cubic structure ...
Text Solution
|
- NH(4)Cl crystallises in a body centred cubic lattice with a unit cell ...
Text Solution
|
- A solid A^(+)B^(-) has NaCl type close packed structure. If the anion ...
Text Solution
|
- Calculate the void space in a primitive unit cell and also the fractio...
Text Solution
|
- Al crystallises in cubic shape unit cell and with edge length 405pm an...
Text Solution
|
- CdO has NaCl structures with density 8.27 g//c c .If the ionic radius...
Text Solution
|
- Gold has a close-packed structure which can be viewed as-spheres occup...
Text Solution
|
- An element has a bcc structure with a cell edge of 288 pm. The density...
Text Solution
|
- When heated above 916^(@)C, iron changes, its crystal structure from b...
Text Solution
|
- Iron occurs as BCC as well as FCC unit cell. If the effective radius o...
Text Solution
|
- Silver has a cubic unit cell with a cell edge of 408 pm. Its density i...
Text Solution
|
- The density of solid argon is 1.65g//mL at -233^(@)C . If the argon a...
Text Solution
|
- An element crystallizes as body - centred cubic lattic. Its density is...
Text Solution
|