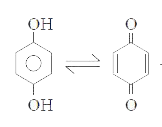
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise ENABLE|50 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise EFFICIENT|46 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise JEE Advanced (Archive)|76 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE)|30 VideosENVIRONMENTAL CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|39 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ELECTROCHEMISTRY-FUNDAMENTAL
- Consider the cell reaction Zn+Cu^(2+)(aq)iff Cu + Zn^(2+) (aq). Reacti...
Text Solution
|
- Cu^(2+) + 2e^(-) rightarrow Cu. For the this, graph betweenE(red) vers...
Text Solution
|
- For the half cell, +2H^(+)2e^(-).E^(0)= 1.30 V. At pH = 2, elec...
Text Solution
|
- In acid medium, MnO(4)^(-) is an oxidising agent ? MnO(4)^(-) + 8H^(...
Text Solution
|
- 1 g equivalent of Na metal is formed from electrolysis of fused NaCl. ...
Text Solution
|
- What will be the emf for the given cell ? Pt|H(2)(g,P(1))|H^(+)(aq)|...
Text Solution
|
- In the following electrochemical cell : Zn(s)abs(zn^(+2)) abs(H^(+))...
Text Solution
|
- If the Pb^(2+) ion concentration is maintained at 1.0 M , what is the ...
Text Solution
|
- Pt(H(2),"x atm")abs(0.0MH^(+))abs(0.1MH^(+))Pt(H(2),"y atm"). if E^(o...
Text Solution
|
- During discharging of lead-storage acid battery following reaction tak...
Text Solution
|
- The cell constant is the product of resistance and
Text Solution
|
- When an electric current is passed through a cell having an electrolyt...
Text Solution
|
- Standard reduction potential at 25^(@)C of Li^(+)//Li,Ba^(+)//Ba,Na^(+...
Text Solution
|
- A conductance cell was filled with a 0.02 M KCl solution which has a s...
Text Solution
|
- The resistance of 1N solution of acetic acid is 250 ohm, when measured...
Text Solution
|
- The single electrode potential E(M^(+)//M) of 0.1 M solution of M^(+) ...
Text Solution
|
- The cell emf for the cell Ni(s)abs(Ni^(2+)(1.0M))Au^(3+)(1.0M)| Au(s) ...
Text Solution
|
- The emf of the cell Cr(s) abs(Cr^(3+)(1.0M))abs(Co^(2+)(1.0M))Co(s) [E...
Text Solution
|
- The reaction 1//2H2(g)+AgCl(s) rarr H^(o+)(aq)+Cl^(c-)(aq)+Ag(s) oc...
Text Solution
|
- The electrolytic cell, one containing acidified ferrous chlo- ride and...
Text Solution
|