A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise IMPECCABLE|50 VideosELECTROCHEMISTRY
VMC MODULES ENGLISH|Exercise ENABLE|50 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VMC MODULES ENGLISH|Exercise JEE ADVANCE (ARCHIVE)|30 VideosENVIRONMENTAL CHEMISTRY
VMC MODULES ENGLISH|Exercise JEE Main (Archive)|39 Videos
Similar Questions
Explore conceptually related problems
VMC MODULES ENGLISH-ELECTROCHEMISTRY-EFFICIENT
- Cathode is made of ......... in a mercury battery:
Text Solution
|
- A current of 5.00 A flowing for 30.00 min deposits 3.048 g of zinc at...
Text Solution
|
- How many coulomb are required for the oxidation of 1 mol of H(2)O(2) t...
Text Solution
|
- In the electroefining of metals, impure metal is
Text Solution
|
- Electrochemical equivalent of Cu in the reaction Cu^(2+) + 2e^(-) ra...
Text Solution
|
- Cell constant is maximum in case of a:
Text Solution
|
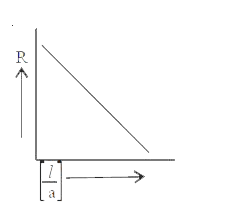
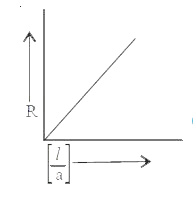
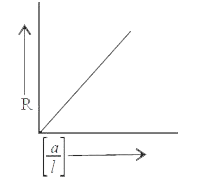
- Variation of resistance with cell constant is given in which of these ...
Text Solution
|
- Ionic conductance of H^(+) and SO(4)""( 2–) at infinite dilution are x...
Text Solution
|
- The equivalent conductance of strong electrolyte:
Text Solution
|
- 100 mL of a buffer of 1 M NH(3)(aq) and 1M NH(4)^(+) (aq) are placed i...
Text Solution
|
- At 25°C the specific conductance of a 1N solution of KCl is 0.002765 m...
Text Solution
|
- On passing electric current of one ampere for 16 min and 5 sec through...
Text Solution
|
- 1 g equivalent of Na metal is formed from electrolysis of fused NaCl. ...
Text Solution
|
- A current was passed for two hour through a solution of an acid that l...
Text Solution
|
- The E^(o) for half cells Fe//Fe^(2+) and Cu//Cu^(2+) are +0.44 V and ...
Text Solution
|
- What is ‘A’ in the following reaction? 2Fe^(3+) (aq) + Sn^(2+) (aq) ...
Text Solution
|
- The E^(o)""(M^(3+)//M^(2+)) values for Cr, Mn, Fe and Co are –0.41, +...
Text Solution
|
- The standard e.m.f of a cell, involving one electron change is found t...
Text Solution
|
- The limiting molar conductivities overset(o)(wedge) for NaCl, KBr an...
Text Solution
|
- When 96500 coulomb of electricity is passed through a copper sulphate ...
Text Solution
|