A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-SOLUTIONS -NEET CAFE (TOPICWISE PRACTICE QUESTIONS)
- Two liquids A and B have vapour pressure in the ratio PA^@: PB^@ = 1:2...
Text Solution
|
- Which among the following is the correct mathematical representation o...
Text Solution
|
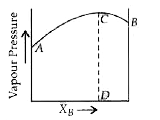
- The diagram given below is a vapour pressure-composition diagram for a...
Text Solution
|
- At a particular temperature, the vapour pressures of two liquids A and...
Text Solution
|
- A solution containing components A and B follows Raoult's law when
Text Solution
|
- In which case Raoult's law is not applicable?
Text Solution
|
- On mixing, heptane and octane form an ideal solution. At 373 K, the va...
Text Solution
|
- Which of the following does not show positive deviation from Raoult's ...
Text Solution
|
- The vapour pressure of pure benzene and toluene are 160 and 60 torr re...
Text Solution
|
- An ideal solution was obtained by mixing methanol and ethanol. If the ...
Text Solution
|
- Benzene and toluene form nearly ideal solutions. At 20 ^@C, the vapour...
Text Solution
|
- A mixture of two completely miscible non-ideal liquids distils as such...
Text Solution
|
- Which of the following pairs shows a negative deviation from Raoult's ...
Text Solution
|
- Among the following substance the lowest vapour pressure is exerted by
Text Solution
|
- Raoult's Law is obeyed by a binary liquid solution when
Text Solution
|
- To 50 mL of water, a few mL of acetone is mixed. The vapour pressure o...
Text Solution
|
- An azeotropic mixture of chloroform and diethyl ether possesses boilin...
Text Solution
|
- Which of the following is satisfied by an ideal solution?
Text Solution
|
- On mixing 25 mL of acetone with 25 mL of ethyl alcohol, the total volu...
Text Solution
|
- Partial pressure of a solution component is directly proportional to i...
Text Solution
|