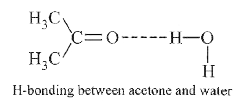A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-SOLUTIONS -NEET CAFE (TOPICWISE PRACTICE QUESTIONS)
- Among the following substance the lowest vapour pressure is exerted by
Text Solution
|
- Raoult's Law is obeyed by a binary liquid solution when
Text Solution
|
- To 50 mL of water, a few mL of acetone is mixed. The vapour pressure o...
Text Solution
|
- An azeotropic mixture of chloroform and diethyl ether possesses boilin...
Text Solution
|
- Which of the following is satisfied by an ideal solution?
Text Solution
|
- On mixing 25 mL of acetone with 25 mL of ethyl alcohol, the total volu...
Text Solution
|
- Partial pressure of a solution component is directly proportional to i...
Text Solution
|
- The vapour pressure of a solution increases when
Text Solution
|
- Which of the following plots does not represent the behaviour of an id...
Text Solution
|
- In a mixture, A and B components show negative deviation
Text Solution
|
- Azeotropic mixture of HCl and H2O has
Text Solution
|
- If H2SO4 is added to water, then the solution
Text Solution
|
- In the mixtures of two miscible volatile liquids obeying Raoults' law,...
Text Solution
|
- In the accompanied diagram the ideal behaviour of a solution is shown ...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- The vapour pressure lowering of a solvent is proportional to
Text Solution
|
- A dilute aqueous solution of glucose shows a vapour pressure of 750 mm...
Text Solution
|
- The freezing point of pure nitrobenzene is 278.8 K. When 2.5 g of unkn...
Text Solution
|
- For which of the following van't Hoff factor cannot be greater than un...
Text Solution
|
- Red blood cell placed in solution having less than 0.91% NaCl will ......
Text Solution
|