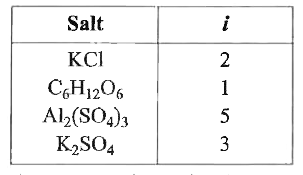
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MTG GUIDE-SOLUTIONS -AIPMT/NEET MCQs
- The van't Hoff factor i for a compound which undergoes dissociation in...
Text Solution
|
- Mole fraction of the solute in a 1.00 molal aqueous solution is
Text Solution
|
- A 0.1 molal aqueous solution of a weak acid is 30% ionised. If Kf for ...
Text Solution
|
- 200 mL of an aqueous solution of a protein contains its 1.26 g. The os...
Text Solution
|
- PA and PB are the vapour pressure of pure liquid components, A and B, ...
Text Solution
|
- Vapour presure of chloroform (CHCl3) and dichloromethane (CH2Cl2) at 2...
Text Solution
|
- How many grams of concentrated nitric acid solution should be used to ...
Text Solution
|
- Of the following 0.10 m aqueous solutions, which one will exhibit the ...
Text Solution
|
- The boiling point of 0.2 mol kg^(-1) solution of X in water is greater...
Text Solution
|
- Which one of the following electrolytes has the same value of van't Ho...
Text Solution
|
- Which of them is not equal to zero for an ideal solution?
Text Solution
|
- What is the mole fraction of the solute in a 1.00 m aqueous solution?
Text Solution
|
- Which of the following statements about the composition of the vapour ...
Text Solution
|
- At 100^@C the vapour pressure of a solution of 6.5 g of a solute in 10...
Text Solution
|
- The van't Hoff factor (i) for a dilute aqueous solution of the strong ...
Text Solution
|
- Which one of the following is incorrect for ideal solution?
Text Solution
|
- If molality of the dilute solution is doubled, the value of molal depr...
Text Solution
|
- Which of the following is dependent on temperature?
Text Solution
|
- For an ideal solution, the correct option is
Text Solution
|
- The mixture that forms maximum boiling azeotrope is
Text Solution
|