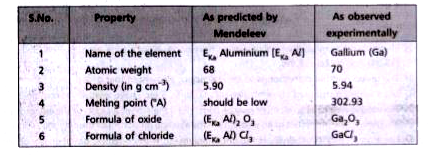
Text Solution
Verified by Experts
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Short Answer Questions|38 VideosCHEMICAL EQUILIBRIUM AND ACIDS BASES
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |31 VideosENVIRONMENTAL CHEMISTRY
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS|6 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-Long Answer Questions
- Discuss in detail about the classification of elements by Mendeleeff.
Text Solution
|
- From a study of properties of neighbouring elements, the properties of...
Text Solution
|
- Define the mordern periodic law . Discuss the construction of t...
Text Solution
|
- Discuss the relation between the number of electron filled into the su...
Text Solution
|
- Write an essay on s, p, d and f-block elements.
Text Solution
|
- Relate the electronic configuration of elements and their properties i...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- What is a periodic property? How the following properties vary in a gr...
Text Solution
|
- Write a note on (a) Atomic radius.
Text Solution
|
- Write a note on (b) Metallic radius.
Text Solution
|
- Write a note on (c ) Covalent radius.
Text Solution
|
- Define IE(1) and IE(2). Why is IE(2) > IE(1) for a given atom? Discuss...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- How do the following properties change in group - 1 and in the third p...
Text Solution
|
- Define electron gain enthalpy. How it varies in a group and in a perio...
Text Solution
|
- What is electronegativity?
Text Solution
|