Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|4 VideosTHERMODYNAMICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Questions|41 VideosTHERMODYNAMICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Very Short Answer Questions|37 VideosTHE S-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |12 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-THERMODYNAMICS-Short Answer Questions
- State the third law of thermodynamics.
Text Solution
|
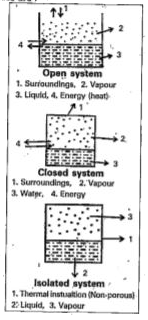
- What are open, closed and isolated systems ? Give one example for each...
Text Solution
|
- Define the state function and state variables. Give examples.
Text Solution
|
- "Internal energy is a state function." Explain.
Text Solution
|
- "Work is not a state function." Explain.
Text Solution
|
- What is heat? Explain.
Text Solution
|
- Derive the equation for 'W("rev")' in isothermal reversible process.
Text Solution
|
- Two litres of an ideal gas at a pressure of 10 atm expands isothermall...
Text Solution
|
- If the ideal gas given in the problem 45 expands against constant exte...
Text Solution
|
- If the ideal gas given in the problem 45 expands to a final volume of ...
Text Solution
|
- Explain the state function 'enthalpy, H'. What is the relationship bet...
Text Solution
|
- Show that DeltaH=DeltaU+Deltan((g)),RT
Text Solution
|
- If water vapour is assumed to be a perfect gas, molar enthalpy change ...
Text Solution
|
- Explain extensive and intensive properties.
Text Solution
|
- Define heat capacity. What are C(p) and C(v)? Show that C(p)-C(v)=R.
Text Solution
|
- Explain the determination of DeltaU of a reaction calorimetrically.
Text Solution
|
- Explain the determination of DeltaH of a reaction calorimetrically:
Text Solution
|
- What is enthalpy of a reaction? Explain the standard enthalpy of a rea...
Text Solution
|
- What is the standard enthalpy of formation? Explain it with example.
Text Solution
|
- Define and explain enthalpy of phase transformation.
Text Solution
|