Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Long Answer Questions|4 VideosTHERMODYNAMICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Important Questions|41 VideosTHERMODYNAMICS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise Very Short Answer Questions|37 VideosTHE S-BLOCK ELEMENTS
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)|Exercise LONG ANSWER QUESTIONS |12 Videos
Similar Questions
Explore conceptually related problems
VIKRAM PUBLICATION ( ANDHRA PUBLICATION)-THERMODYNAMICS-Short Answer Questions
- Define heat capacity. What are C(p) and C(v)? Show that C(p)-C(v)=R.
Text Solution
|
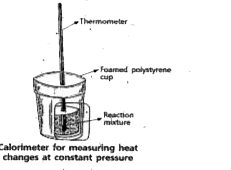
- Explain the determination of DeltaU of a reaction calorimetrically.
Text Solution
|
- Explain the determination of DeltaH of a reaction calorimetrically:
Text Solution
|
- What is enthalpy of a reaction? Explain the standard enthalpy of a rea...
Text Solution
|
- What is the standard enthalpy of formation? Explain it with example.
Text Solution
|
- Define and explain enthalpy of phase transformation.
Text Solution
|
- Define and explain the standard enthalpy of vapourisation (Molar entha...
Text Solution
|
- Define and explain the standard enthalpy of vapourisation (Molar entha...
Text Solution
|
- Define and explain the standard enthalpy of vapourisation of sublimati...
Text Solution
|
- Define and explain the standard enthalpy of formation (Delta(r)H^(thet...
Text Solution
|
- State and explain the Hess's law of constant heat summation.
Text Solution
|
- Define and explain the enthalpy of combustion (Delta(c)H^(theta)).
Text Solution
|
- Define and explain the enthalpy of atomisation(Delta(a)H^(theta)).
Text Solution
|
- Define and explain the bond enthalpy (Delta("bond")H^(theta)).
Text Solution
|
- What is the bond enthalpy of C-H bond of CH(4)?
Text Solution
|
- Define heat of solution (Delta("sol")H^(theta)) and heat of dilution.
Text Solution
|
- Define ionisation enthalpy and electron affinity.
Text Solution
|
- Explain the spontaneity of a process.
Text Solution
|
- Is decrease in enthalpy a criterion for spontaneity? Explain.
Text Solution
|
- What is entropy? Explain with examples.
Text Solution
|