Text Solution
Verified by Experts
Topper's Solved these Questions
METALS AND NON METALS
ZEN PUBLICATION|Exercise QUESTIONS SECTION (TEXTUAL EXERSCISE)|16 VideosMETALS AND NON METALS
ZEN PUBLICATION|Exercise ZEN ADDITIONAL QUESTIONS SECTION (MULTIPLE CHOICE QUESTIONS)|31 VideosCHEMICAL REACTIONS AND EQUATIONS
ZEN PUBLICATION|Exercise ZEN ADDITIONAL QUESTIONS SECTION (LONG - ANSWER [LA]TYPE QUESTIONS )|27 VideosPERIODIC CLASSIFICATION OF ELEMENTS
ZEN PUBLICATION|Exercise QUESTIONS|2 Videos
Similar Questions
Explore conceptually related problems
ZEN PUBLICATION-METALS AND NON METALS-ZEN ADDITIONAL QUESTIONS SECTION (LONG ANSWER [LA] TYPE 2 QUESTIONS)
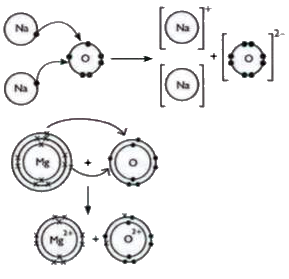
- (i) Write the electron dot structure for sodium, oxygen and magnesium....
Text Solution
|
- State one property of each of the following which makes them suitable ...
Text Solution
|
- a. When calcium metal is added to water, the gas evolved does not catc...
Text Solution
|
- Describe how sodium and chlorine form sodium chloride. Name the type o...
Text Solution
|
- Describe general properties of ionic compounds.
Text Solution
|
- Write balanced chemical equations for the reactions taking place when ...
Text Solution
|
- A metal X is stored under kerosene. When a small piece of it is left o...
Text Solution
|
- a. The reaction of metal E with ferric oxide is hightly exothermic. Me...
Text Solution
|
- Carbon cannot be used to reduce the oxide of sodium, magnetsium, calci...
Text Solution
|
- (a) Most of the metals acquire a dull surface when exposed to air . Na...
Text Solution
|
- Give an account of the methods to prevent corrosion of metals.
Text Solution
|
- a.Define activity series of metals. Arrange the metals Gold, Copper, i...
Text Solution
|
- Mention the difference between calcination and roasting. How these pro...
Text Solution
|
- a.Give two methods to prevent the rusting to iron. b. Name theores o...
Text Solution
|
- a. Define corrosion. b. What is corrosion of iron called? c.Why cor...
Text Solution
|