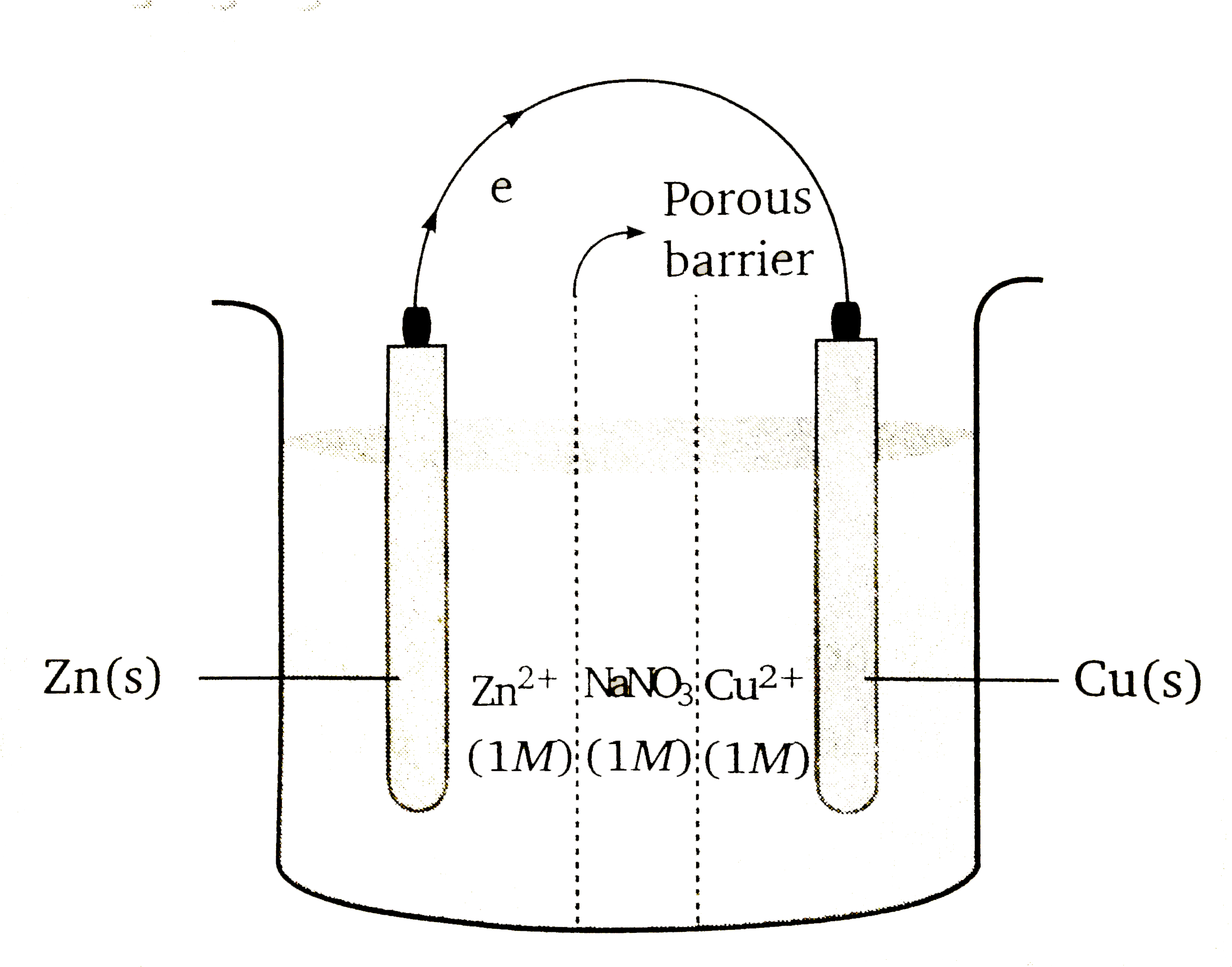A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - One Or More Answers Are Correct|3 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|1 VideosELECTROCHEMISTRY
NARENDRA AWASTHI|Exercise Level 2|2 VideosDILUTE SOLUTION
NARENDRA AWASTHI|Exercise Level 3 - Match The Column|1 VideosGASEOUS STATE
NARENDRA AWASTHI|Exercise Level 3 - Subjective Problems|1 Videos
Similar Questions
Explore conceptually related problems
NARENDRA AWASTHI-ELECTROCHEMISTRY-Level 3 - Passage
- A Galvanic cell consits of three compartment as shown in figure. The f...
Text Solution
|
- Calculate the oxidation state of nitrogen ( N ) in NH3
Text Solution
|
- Write formula for the following compounds : Mercury(II)chloride
Text Solution
|
- The molar conductivity of 0.04 M solution of MgCl2 is 200 Scm^2mol^(-1...
Text Solution
|
- A saturated solution in AgX(K(sp)=3xx10^(-12)) and AgY(K(sp)=10^(-12)...
Text Solution
|