10 mL of `H_(2)A`(weak diprtic acid) solutio is titrated against 0.1M NaOH. pH of the solution is plotted against volume of strong base added and following obserbation is made
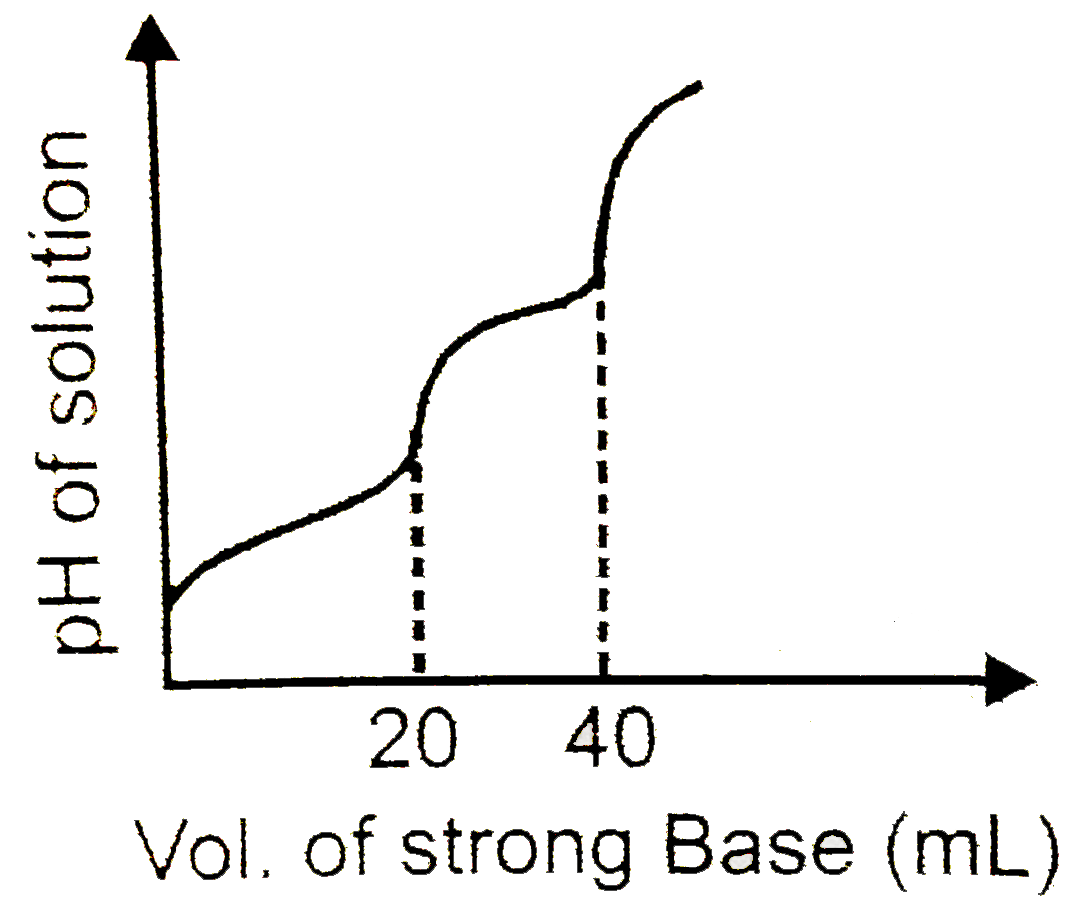
Ip pH of the solution at first equivalence point is `pH_(1)` and at secnd equibalence point is `pH_(2^.) Calculate the value of `(pH_(2)-pH_(1))` at `25^(@)C`
Given for `H_(2)A,pK_(a_1)` =4.6 and `pK_(a_2)` =8, log 25=1.4