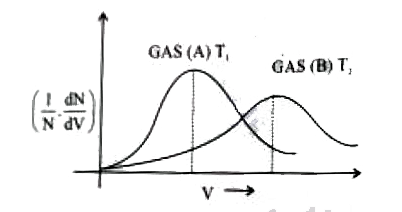A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-STATES OF MATTER-NEET PAST 5 YEARS QUESTIONS
- According to Maxwell.s distribution of molecular speeds, for the below...
Text Solution
|
- A mixture of N2 and Ar gases in a cylinder contains 7g of N2 & 8 g of ...
Text Solution
|
- The minimum pressure required to compress 600 dm^3 of a gas at 1 bar t...
Text Solution
|
- A gas at 350 K and 15 bar has molar volume 20 percent smaller than tha...
Text Solution
|
- The correction factor 'a' to the ideal gas equation corresponds to
Text Solution
|
- Given van der Waals constant for NH(3), H(2), O(2) and CO(2) are respe...
Text Solution
|
- A 20 litre container at 400 K contains CO(2) (g) at pressure 0.4 atm a...
Text Solution
|
- Equal moles of hydrogen and oxygen gases are placed in a container wit...
Text Solution
|
- A mixture of N2 and Ar gases in a cylinder contains 7g of N2 & 8 g of ...
Text Solution
|
- The minimum pressure required to compress 600 dm^3 of a gas at 1 bar t...
Text Solution
|
- A gas at 350 K and 15 bar has molar volume 20 percent smaller than tha...
Text Solution
|
- The correction factor 'a' to the ideal gas equation corresponds to
Text Solution
|
- Given van der Waals constant for NH(3), H(2), O(2) and CO(2) are respe...
Text Solution
|
- A 20 litre container at 400 K contains CO2(g) at 0.4 atm and an excess...
Text Solution
|
- Equal moles of hydrogen and oxygen gases are placed in a container wit...
Text Solution
|