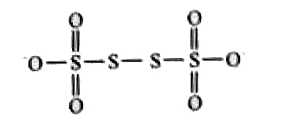
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
PHYSICS WALLAH-REDOX REACTIONS-LEVEL-1
- The oxidation states of S-atoms in S(4)O(6)^(2-) from left to right re...
Text Solution
|
- Which of the following is the most powerful oxidising agent ?
Text Solution
|
- In acidic medium, H(2)O(2) changes Cr(2)O(7)^(-2) to CrO(5) which has ...
Text Solution
|
- Which of the following is a redox reaction?
Text Solution
|
- The volume of 0.1M oxalic acid that can be completely oxidised by 20mL...
Text Solution
|
- In the following reaction: 3Fe+4H(2)OrarrFe(3)O(4)+4H(2), if the ato...
Text Solution
|
- The oxidation number and covalency of sulphur in the sulphur molecule ...
Text Solution
|
- Oxidation state of P in H(4)P(2)O(5), H(4)P(2)O(6), H(4)P(2)O(7) are r...
Text Solution
|
- Oxidation number if iodine in IO(3)^(-), IO(4)^(-),KI and I(2) respect...
Text Solution
|
- Which of the following have been arranged in the decreasing order of o...
Text Solution
|
- Carbon is in the lowest oxidation state in
Text Solution
|
- Which of the following can act as oxidising as well as reducing agent?
Text Solution
|
- A mixture of potassium chlorate, oxalic acid and sulphuric acid is hea...
Text Solution
|
- How many moles of iodine are liberated when 1 m potassium dichromate r...
Text Solution
|
- Oxidation number of 'N' in N(3)H (hydrazoic acid) is :-
Text Solution
|
- Which one of the following is not a redox reaction :-
Text Solution
|
- Which of the following examples does not represent disproportionation ...
Text Solution
|
- The oxidation number and covalency of sulphur in the sulphur molecule ...
Text Solution
|
- In a reaction, H(2)O +C rarr CO +H(2)
Text Solution
|
- 0.52 g of a dibasic acid required 100 mL of 0.2 N NaOH for complete ne...
Text Solution
|
- In which of the following compound oxidation number of Cl is +3?
Text Solution
|