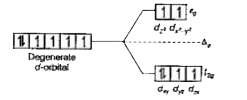
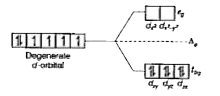
Text Solution
Verified by Experts
Topper's Solved these Questions
COORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part II Short Answer Type II Questions 3 Marks |12 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part II Long Answer Type Questions 7 Marks |9 VideosCOORDINATION COMPOUNDS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE Part II Very Short Answer Type Questions 1 Mark |6 VideosCHEMISTRY IN EVERYDAY LIFE
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS) |2 VideosD-BLOCK ELEMENTS
ARIHANT PUBLICATION|Exercise Chapter practice (Long answer type questions)|2 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-COORDINATION COMPOUNDS -QUESTIONS FOR PRACTICE Part II Short Answer Type I Questions 2 Marks
- Identify A and B in the given sequence of reaction. Also, write their ...
Text Solution
|
- Why do compounds having similar geometry have different magnetic momen...
Text Solution
|
- On the basis of crystal field theory explain why Co(III) forms paramag...
Text Solution
|
- Give the oxidation state, d-orbital occupation and coordination number...
Text Solution
|
- Give the oxidation state, d-orbital occupation and coordination number...
Text Solution
|
- Give the oxidation state, d-orbital occupation and coordination number...
Text Solution
|
- Give the oxidation state, d-orbital occupation and coordination number...
Text Solution
|
- Why are different colours observed in octahedral and tetrahedral compl...
Text Solution
|
- What will be the correct order for the wavelength of absorption in the...
Text Solution
|