Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES (ALKYL HALIDE)
ARIHANT PUBLICATION|Exercise PART-I- QUESTION FOR PRACTICE(SHORT ANSWER TYPE-II QUESTIONS)|10 VideosHALOALKANES (ALKYL HALIDE)
ARIHANT PUBLICATION|Exercise PART-I- QUESTION FOR PRACTICE(LONG ANSWER TYPE QUESTIONS)|3 VideosHALOALKANES (ALKYL HALIDE)
ARIHANT PUBLICATION|Exercise PART-I- QUESTION FOR PRACTICE(VERY SHORT ANSWER TYPE QUESTIONS)|4 VideosGROUP 18 ELEMENTS NOBLEGASES
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( Long Answer Type Questions )|6 VideosPHENOLS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions )|1 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-HALOALKANES (ALKYL HALIDE) -PART-I- QUESTION FOR PRACTICE(SHORT ANSWER TYPE-I QUESTIONS)
- Give the IUPAC names of the following compounds. (CH(3))(3)C CH(2)-C...
Text Solution
|
- Give the IUPAC names of the following compounds. CH(3)CH=undersetun...
Text Solution
|
- Give the IUPAC names of the following compounds. (CH(3))(3)C CH(2)-C...
Text Solution
|
- Give the IUPAC names of the following compounds. (CH(3))(3)C CH(2)-C...
Text Solution
|
- Why is sulphuric acid not used during the reaction of alcohols with KI...
Text Solution
|
- Write the structure of the major organic products in each of the follo...
Text Solution
|
- Write the structure of the major organic products in each of the follo...
Text Solution
|
- Write the structure of the major organic products in each of the follo...
Text Solution
|
- Write the structure of the major organic products in each of the follo...
Text Solution
|
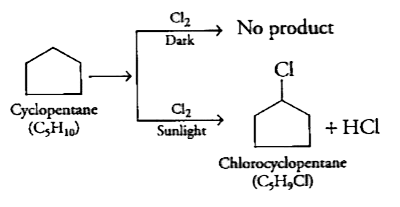
- A hydrocarbon C(5)H(10) does not react with chlorine in dark but gives...
Text Solution
|