Text Solution
Verified by Experts
Topper's Solved these Questions
PHENOLS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (Short Answer Type II Questions )|2 VideosPHENOLS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (Long Answer Type Questions)|3 VideosPHENOLS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE (Very Short Answer Type Questions)|12 VideosHALOALKANES (ALKYL HALIDE)
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (LONG ANSWER TYPE QUESTIONS)|5 VideosPOLYMERS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE ( LONG ANSWER TYPE QUESTIONS )|3 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-PHENOLS -QUESTIONS FOR PRACTICE (Short Answer Type 1 Questions)
- Give the structures and IUPAC names of monohydric phenols of molecula...
Text Solution
|
- How can you convert benzene to phenol?
Text Solution
|
- How is phenol prepared? Give equation.
Text Solution
|
- Arrange the following compounds in the increasing order of acidity an...
Text Solution
|
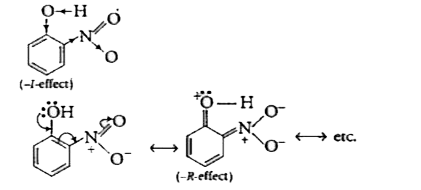
- Ortho and para-nitrophenols are more acidic than phenol. Draw the reso...
Text Solution
|
- Explain why p-nitrophenol is more acidic than phenol?
Text Solution
|
- What happens when phenol is treated with chloroform and dilute alkali...
Text Solution
|
- What is Reimer-Tiemann reaction?
Text Solution
|
- Explain why phenol is acidic while C2H5 OH is neutral?
Text Solution
|
- How will you convert phenol to 2,4,6-tribromo phenol?
Text Solution
|
- Suggest two tests to distinguish between ethanol and phenol.
Text Solution
|
- What is Reimer-Tiemann reaction?
Text Solution
|