Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-QUESTION PAPER 2019-GROUP B
- Explain what are ionic and covalent solids.Give one example of each.
Text Solution
|
- An organic compound having molecular formula C3H7Br on treatment with ...
Text Solution
|
- Answer the following What is the action of chlorine with (i) cold ...
Text Solution
|
- Answer the following What do you mean by biodegradale and non-biodeg...
Text Solution
|
- Discuss Reimer-Tiemann reaction.
Text Solution
|
- Write a note on hydrogen-oxygen fuel cell.
Text Solution
|
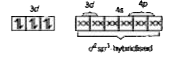
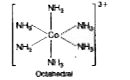
- Discuss the struchure of [Co(NH3)6]^(3+) ion on the basis of valence b...
Text Solution
|
- The rate constants ofa reaction at 500 K and 700 K are 0.025 sec^-1 an...
Text Solution
|
- Elucidate the differences between soaps and detergents.
Text Solution
|
- Answer any seven questions of the following Discuss van Arkel Boer m...
Text Solution
|
- The osmotic pressure ofa solution containing 50 g of a solute in one l...
Text Solution
|
- Answer the following: Match the diseases of Group (A) with the vitam...
Text Solution
|
- Answer any seven questions of the following: What are bidentate liga...
Text Solution
|
- Answer any seven questions of the following: What happens when yello...
Text Solution
|
- What are freons ? What are their harmful effects on the environment ?
Text Solution
|
- How does Schottky defect arise ? In which type of ionic compounds does...
Text Solution
|
- Answer any seven questions of the following: CuSO4 solution is elect...
Text Solution
|
- Answer any seven questions of the following: Under which condition ...
Text Solution
|
- What happens when KI solution is added to acidified K2Cr2O7 solution?
Text Solution
|
- What are antioxidants ? Give two examples.
Text Solution
|