A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion - Reason Type)|20 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS|11 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise LEVEL-I|50 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-III|52 VideosENVIRONMENTAL CHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTIONS LEVEL-II (ASSERTION-REASON TYPE)|10 Videos
BRILLIANT PUBLICATION-CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES-LEVEL-II
- The correct order of increasing ionic character is
Text Solution
|
- Ionisation potential and electron affinity of fluorine are 17.42 and 3...
Text Solution
|
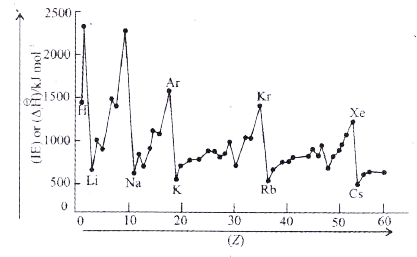
- The graph of IE(1) or DeltaH(1)Ɵ versus atomic number (Z) is given bel...
Text Solution
|
- Elements a, b, c, d and e have the following electronic configuration ...
Text Solution
|
- Which of the following order is wrong?
Text Solution
|
- General electronic configuration of outermost and penultimate shell of...
Text Solution
|
- An element X belongs to fourth period and fifteenth group of the perio...
Text Solution
|
- The correct order of decreasing first ionization energy is:
Text Solution
|
- Similarity in chemical properties of the atoms of elements in a group ...
Text Solution
|
- In the long form of the periodic table, the valence shell electronic c...
Text Solution
|
- In which of the following sets of atomic numbers, all the elements do ...
Text Solution
|
- Which of the following element is just below in the E.C. of [Ne]3s^(2)...
Text Solution
|
- Chloride of an element A gives neutral solution in water. In the perio...
Text Solution
|
- Write the period number, group number and block of the element having ...
Text Solution
|
- Among the elements with the following atomic numbers, which are d-bloc...
Text Solution
|
- If the atomic number of an inert gas element is Z, then an element wit...
Text Solution
|
- The process requiring absorption of energy is
Text Solution
|
- Which of the following is correct regarding the variation of propertie...
Text Solution
|
- The electronegativity of H, O and X are 2.1, 3.5 and 0.7 respectively....
Text Solution
|
- ns^(2) np^(4) (n-outermost orbit) represents the valence electrons. T...
Text Solution
|