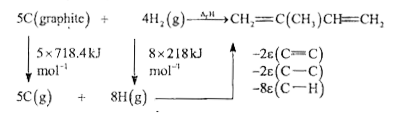A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-II (Assertion-Reason Type)|18 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-III|52 VideosCHEMICAL THERMODYNAMICS
BRILLIANT PUBLICATION|Exercise LEVEL-I|92 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
BRILLIANT PUBLICATION|Exercise QUESTION (LEVEL -ll)|42 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES
BRILLIANT PUBLICATION|Exercise QUESTIONS (LEVEL-III ( Linked Comprehension Type ))|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-CHEMICAL THERMODYNAMICS -LEVEL-II
- If Delta (f) H ^(@) (CO,g) =- 110.5 kJ mol ^(-1) and Delta (f) H ^(@) ...
Text Solution
|
- The enthalpy of combustion of H(2)(g) at 298 K to give H(2)O(g) is -24...
Text Solution
|
- If bond enthalpies of (C- H) = 413 kJ mol ^(-1) , (C -C) = 347. 7 kJ m...
Text Solution
|
- If Delta (f) G ^(@) (H(2)O, l) = - 237 . 19 kJ mol ^(-1) and Delta (f...
Text Solution
|
- 36 mL of pure water takes 100 sec to evaporate from a vessel and heate...
Text Solution
|
- Consider the reaction at 300 K, C (6) H (6) (l) + (15)/(2) O (2) (g) t...
Text Solution
|
- A rigid and insulated tank of 3 m^3 volume is divided into two compart...
Text Solution
|
- The enthalpy of neutralization of a weak monoprotic acid (HA) in 1 M s...
Text Solution
|
- Assertion : Work done during free expansion of an ideal gas whether re...
Text Solution
|
- Assertion : The solubility of most salts in water increases with rise ...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- How many joules of heat are absorbed when 70.0 g of water is completel...
Text Solution
|
- An ideal gas is allowed to expand against a constant pressure of 2 bar...
Text Solution
|
- The enthalpy of reaction for the reaction: 2H (2 (g)) + O (2 (g)) to 2...
Text Solution
|
- In a calorimeter, the temperature increases by 6.12 K, the heat capaci...
Text Solution
|
- Equal volumes of monoatomic and diatomic gases at same initial tempera...
Text Solution
|
- The heat liberated from the combustion of 0.5 g of carbon raised the t...
Text Solution
|
- The enthalpy change for the reaction, N2(g)+3H2(g)rightarrow2NH3(g) ...
Text Solution
|
- The specific heats of l(2) in vapour and solid states are 0.031 and 0....
Text Solution
|
- Temperature of I mole of a gas is increased by 1^(@)at constant pressu...
Text Solution
|