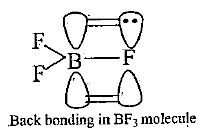In `BF_3`, boron is `sp^3` hybridised. It has a vacant 2p-orbital. Each fluorine in `BF_3` has completely filled unutilized 2p-orbitals. Since, both of these orbitals belong to same energy level, `p pi-p pi` back bonding occurs in which a lone pair of electron-is transferred from unutilized, completely filled 2p-orbital of Fto vacant 2p-orbital of B. This type of bond formation is known as back bonding. Therefore, B-F bond has some double bond character. That.s why all the three boron-fluorine bonds are shorter than the usual single boron-fluorine bond.