A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
BRILLIANT PUBLICATION|Exercise LEVEL-II|50 VideosSOLID STATE
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION - REASON TYPE)|20 VideosSOLID STATE
BRILLIANT PUBLICATION|Exercise QUESTION|14 VideosREDOX REACTION & ELECTROCHEMISTRY
BRILLIANT PUBLICATION|Exercise QUESTION (ELECTROCHEMISTRY) (LEVEL -II) (ASSERTION-REASON)|3 VideosSOLUTIONS
BRILLIANT PUBLICATION|Exercise Level-III (Linked Comprehension Type)|12 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-SOLID STATE-LEVEL-I
- In a solid lattice, the cation has left a lattice site and is located ...
Text Solution
|
- If we know the ionic radius ratio in a crystal of ionic solid, what ca...
Text Solution
|
- The number of nearest neighbours with which a given sphere, of packing...
Text Solution
|
- Total volume of atoms present in face centred cubic unit cell of a met...
Text Solution
|
- Percentage of free space in cubic close-packed structure and in body-c...
Text Solution
|
- How many octahedral voids are there in 1 mole of a compound having cu...
Text Solution
|
- Crystal defect indicated in the diagram below is:
Text Solution
|
- Frenkel defect is noticed in
Text Solution
|
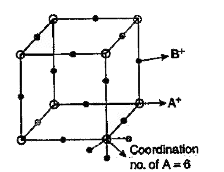
- The arrangement of X^(-) ions around A^(+) ion in solid AX is given in...
Text Solution
|
- Which of the following fcc structure contains cations in the alternate...
Text Solution
|
- CsBr has bcc type structure like CsCl with edge length 4.3 Å. The shor...
Text Solution
|
- The C0ordination number of a metal crystallizing in a hexagonal close ...
Text Solution
|
- A crystal formula AB(3) has A ions at the cube corners and B ions at t...
Text Solution
|
- What type of crystal defect is produced when ' NaCl' is:doped with 'Mg...
Text Solution
|
- The lattice parameters are a=5.62A^(@),b=7.41A^(@),c=9.48A^(@). The th...
Text Solution
|
- At a temperature of absolute zero, an intrinsic semiconductor is
Text Solution
|
- The ratio Fe^(3+) and Fe^(2+) ions in Fe(0.9)S(1.0) is.
Text Solution
|
- The yellow colour of ZnO and conducting nature produced in heating is ...
Text Solution
|
- In an antiferromagnetic material
Text Solution
|
- Which of the following is not a ferroelectric compound?
Text Solution
|