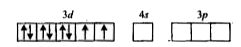
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION- REASON TYPE )|20 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL I |55 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL - I|55 VideosCO-ORDINATION COMPOUNDS AND ORGANOMETALLICS
BRILLIANT PUBLICATION|Exercise Level-II (Assertion- Reason)|4 VideosD & F BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise Level -II|38 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-COORDINATION COMPOUNDS -LEVEL-II
- Among [Ni(CO)4], [NiCl4]^(2-), [Co(NH3)4 Cl2] Cl, Na3 [CoF6], Na2O2 an...
Text Solution
|
- Which of these statements about [Co(CN)6]^(3-) is true?
Text Solution
|
- [NiCl2 {P(C2H5)2(C6H5)}2] exhibits temperature dependent magnetic beha...
Text Solution
|
- The octahedral complex of a metal ion , M^(3+), with four monodentate ...
Text Solution
|
- If the length of CO bond in carbon monoxide is. 1.128 Å, then what is ...
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Dipole moment will be zero in the complexes I. [Ni(CN)4]^(2-) II. ci...
Text Solution
|
- In nitroprusside ion, iron and NO exist as Fe (II) and NO^(+) rather t...
Text Solution
|
- Which of the following statements is/are correct for [Cr(NH3)5 Br] SO4...
Text Solution
|
- A solution containing 2.675g of CoCl3. 6NH3 (molar mass=267.5 g mol^(-...
Text Solution
|
- One mole of the coinplex compound Co(NH3)5 Cl3 gives three moles of io...
Text Solution
|
- Which of the following name formụla combinations is not correct?
Text Solution
|
- What is the correct name for the following complex? [Co(NH2)2(NH2 -CH3...
Text Solution
|
- The no.of geometrical isomers that can exist for the square planar com...
Text Solution
|
- Which of the electrònic configuration according to crystál field theor...
Text Solution
|
- Which of the following complex is heteroleptic as well as unable to sh...
Text Solution
|
- Ammonia forms the complexion [Cu(NH3)4]^(2+) with copper ions in alkal...
Text Solution
|
- Nickel (Z = 28) combines with a uninegative monodentate ligand X^(-) t...
Text Solution
|
- The correct order of magnetic moments (spin only values in B.M.) among...
Text Solution
|
- Which of the following options is correct when six ligands are approac...
Text Solution
|