A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
COORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL-II (ASSERTION- REASON TYPE )|20 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL I |55 VideosCOORDINATION COMPOUNDS
BRILLIANT PUBLICATION|Exercise LEVEL - I|55 VideosCO-ORDINATION COMPOUNDS AND ORGANOMETALLICS
BRILLIANT PUBLICATION|Exercise Level-II (Assertion- Reason)|4 VideosD & F BLOCK ELEMENTS
BRILLIANT PUBLICATION|Exercise Level -II|38 Videos
Similar Questions
Explore conceptually related problems
BRILLIANT PUBLICATION-COORDINATION COMPOUNDS -LEVEL-II
- The no.of geometrical isomers that can exist for the square planar com...
Text Solution
|
- Which of the electrònic configuration according to crystál field theor...
Text Solution
|
- Which of the following complex is heteroleptic as well as unable to sh...
Text Solution
|
- Ammonia forms the complexion [Cu(NH3)4]^(2+) with copper ions in alkal...
Text Solution
|
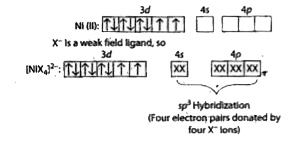
- Nickel (Z = 28) combines with a uninegative monodentate ligand X^(-) t...
Text Solution
|
- The correct order of magnetic moments (spin only values in B.M.) among...
Text Solution
|
- Which of the following options is correct when six ligands are approac...
Text Solution
|
- Two compounds Co(NO2)3 and KNO2 are mixed together in 1 : 3 proportion...
Text Solution
|
- Select the true statement from the following for metal carbonyls?
Text Solution
|
- Which of the following complexes has magnetic moment of 2.83 Bohr magn...
Text Solution
|
- Which of the following statement is not correct?
Text Solution
|
- Relative to the average energy in the spherical crystal field, the eg ...
Text Solution
|
- Of the following statements, which one is correct?
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- The complexes [FeCl4]^(2-), [CoCl4]^(2-) and [Cu(CN)4]^(2-) respectiv...
Text Solution
|
- Whịch of the following statements regarding the complex [M(A A)2 a2] i...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- One mole of the coinplex compound Co(NH3)5 Cl3 gives three moles of io...
Text Solution
|
- Of the following complexes, the correct statement is 1. trans-[Co(NH...
Text Solution
|
- The complex [Pt(py) (NH3) (NO2) ClBrl] has
Text Solution
|