A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -GEOMETRICAL OPTICS-EXERCISE -02
- N (lt 100) molecules of a gas have velocities 1,2,3….N km/s respective...
Text Solution
|
- Let barv,v(rms) and vp respectively denote the mean speed. Root mean s...
Text Solution
|
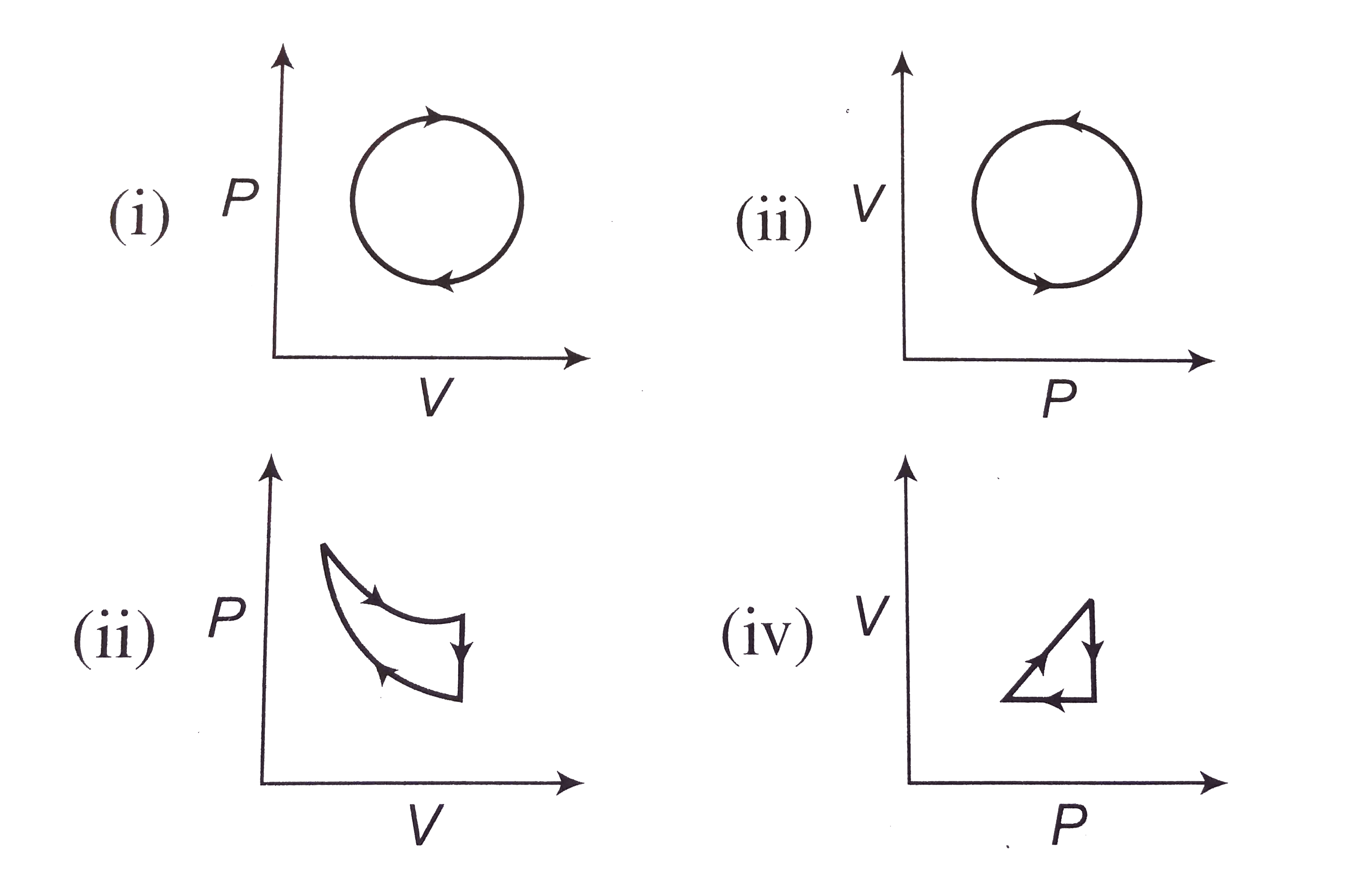
- The following are the P-V diagrams for cyclic processes for a gas. In ...
Text Solution
|
- The internal energy of a system remains constant when it undergoes (...
Text Solution
|
- C(p) is always greater than C(V) due to the fact that:-
Text Solution
|
- An ideal gas is heated from termperature T(1) to T(2) under various co...
Text Solution
|
- The indicator diagram for two processes 1 and 2 carrying on an ideal g...
Text Solution
|
- An ideal monoatomic gas undergoes a cyclic process ABCA as shown in th...
Text Solution
|
- Logarithms of readings of pressure and volume for an ideal gas were pl...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- A thermally insulated chamber of volume 2V(0) is divided by a friction...
Text Solution
|
- One mole of an ideal monoatomaic gas is taken from A to C along the pa...
Text Solution
|
- The thermal capacity of 40 g of aluminium (specific heat =0.2 cal//gm^...
Text Solution
|
- Two cylinders A and B fitted with pistons contain equal amounts of an ...
Text Solution
|
- One mole of an ideal gas at an initial temperature of TK does 6R joul...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- A gas expands such that its initial and final temperatures are equal. ...
Text Solution
|
- A gas takes part in two processes in which it is heated from the same ...
Text Solution
|
- Radiation from a black body at the thermodynamic temperature T(1) is m...
Text Solution
|
- A point source of heat of power P is placed at the centre of a spheric...
Text Solution
|
_E01_090_O01.png)
_E01_090_O02.png)
_E01_090_O03.png)
_E01_090_O04.png)