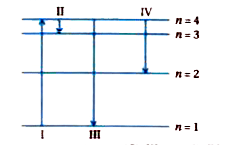
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise-02|19 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise Exercise- 3 Match The Column|1 VideosSIMPLE HARMONIC MOTION
ALLEN |Exercise SOME WORKED OUT EXAMPLES|29 VideosRACE
ALLEN |Exercise Basic Maths (Wave Motion & Dopplers Effect) (Stationary waves & doppler effect, beats)|25 VideosTEST PAPER
ALLEN |Exercise PHYSICS|4 Videos
Similar Questions
Explore conceptually related problems
ALLEN -SIMPLE HARMONIC MOTION-Exercise-01
- If elements with principal quantum number ngt 4 were not allowed in na...
Text Solution
|
- If 13.6 eV energy is required to ionized the hydrogen atom, then the e...
Text Solution
|
- The diagram shows the energy levels for an electron in a certain atom....
Text Solution
|
- The muon has the same change as an electron but a mass that is 207 tim...
Text Solution
|
- In problems involving electromagenetism it is often convenlent and inf...
Text Solution
|
- Which of the following transitions in hydrogen atoms emit photons of h...
Text Solution
|
- The velocity of an electron in single electron atom in an orbit
Text Solution
|
- The energy level diagram is for a hypotenitcal atom. A gas of these at...
Text Solution
|
- The wave number of the series limit of Lyman series is -
Text Solution
|
- In a hypothetical system , a particle of mass m and charge -3 q is mov...
Text Solution
|
- The diagram to the right shows the lowest four energy levels for an el...
Text Solution
|
- An energy of 24.6 eV is required to remove one of that electrons from ...
Text Solution
|
- A photons was absorbed by a hydrogen atom in its ground state, and the...
Text Solution
|
- The K(alpha) X-ray emission line of tungsten occurs at lambda = 0.021 ...
Text Solution
|
- Which of the following are the characteristics required for the target...
Text Solution
|
- The wavelength of K(alpha) line for an element of atomic number 43 is ...
Text Solution
|
- The wavelength of K(alpha) X-rays of two metals A and B are 4 // 1875 ...
Text Solution
|
- On operating an X-ray tube at 1 kV. X-rays of minimum wavelength 6.22Å...
Text Solution
|
- In an X-rays tube, electrons striking a target are brought to rest, ca...
Text Solution
|
- The production of characteristic X-rays is due to-
Text Solution
|