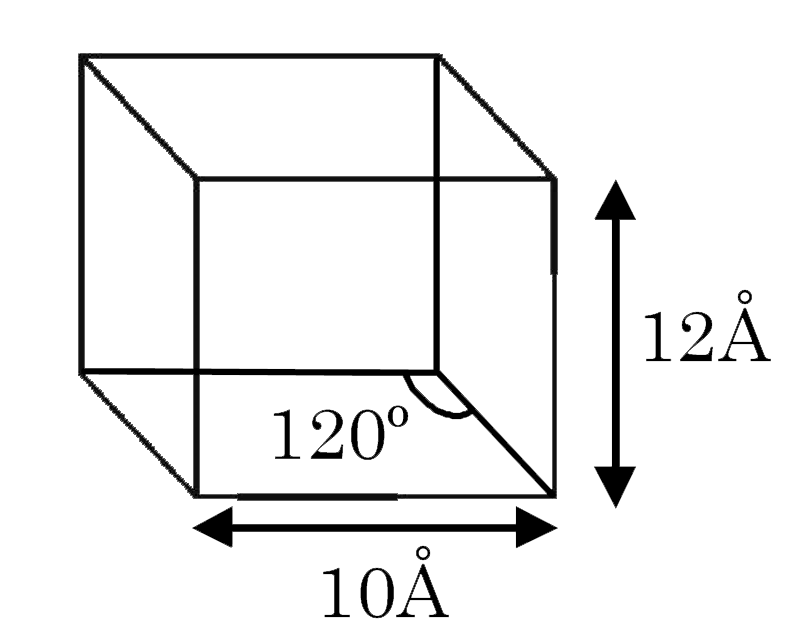
A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -TEST PAPERS-CHEMISTRY
- The least positive value of theta that satisfies 1/(cos5theta)=1/(cost...
Text Solution
|
- The complete solution of |x+1|+|x-4|-gt7 is (-oo,-a)uu(b,oo) where a ...
Text Solution
|
- Compound A(2)B crystallise in hexagonal lattice at low temperature. Un...
Text Solution
|
- Given: C(2)H(2)(g)+2H(2)(g)toC(2)H(6)(g) At 400K it was observed du...
Text Solution
|
- Whch of the following statement in INCORRCT about CO(3)^(2-) ion?
Text Solution
|
- Bond energy is maximum for
Text Solution
|
- Consider following structures and select INCORRECT order.
Text Solution
|
- Which of the following pairs are resonance structures of each other?
Text Solution
|
- Identify compound incorrectly match with total number of Deuterium exc...
Text Solution
|
- Which is/are correct statements about a solution containing CH(3)COCH(...
Text Solution
|
- Which is /are correct statements about face centered cubic unit cell o...
Text Solution
|
- Consider following combintion reactions. and select CORRECT optio...
Text Solution
|
- Which of the following statement is/are INCORRECT?
Text Solution
|
- Consider given structure of silicate anion. and find correct stat...
Text Solution
|
- Correct order of stability is/are:
Text Solution
|
- Which of the following compound(s)have C-N bond length is more than C-...
Text Solution
|
- Liquid A(P(A)^(0)=100mm Hg) and liquid B(P(B)^(0)=400mmHg) from an ide...
Text Solution
|
- Liquid A(P(A)^(0)=100mm Hg) and liquid B(P(B)^(0)=400mmHg) from an ide...
Text Solution
|
- Acid strength refers to the tendency of an acid (HA) to dissociate int...
Text Solution
|
- Acid strength refers to the tendency of an acid (HA) to dissociate int...
Text Solution
|