A
B
C
D
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN -TEST PAPERS-CHEMISTRY
- A constant force acting at the centre of a uniform disk of radius r is...
Text Solution
|
- One mole of hydrogen assumed to be ideal, is adiabatically expanded fr...
Text Solution
|
- An ideal gas undergoes a cycle ABCA in which its pressure P, and volum...
Text Solution
|
- For an elementary reaction A(g)+B(g)+C(g)to2D(g) initial partial p...
Text Solution
|
- In a non stoichiometric compound Ni(0.9)O. Find the ratio of Ni^(2+) &...
Text Solution
|
- Volume vs Temperature graph of 3 moles of diatomic gas is as shown in ...
Text Solution
|
- Which one of the following property is amount independent
Text Solution
|
- In a facc lattice of atoms the number of atoms in one unit cell that a...
Text Solution
|
- In which of the following 3c-4e bond is present?
Text Solution
|
- Given the correct order of initials T or F for the following statement...
Text Solution
|
- Which is NOT correctly matched?
Text Solution
|
- Select the INCORRECT order.
Text Solution
|
- Consider the following conversions: N(2)toN(2)^(+) O(2) to O(2)^(+...
Text Solution
|
- Optically inactive molecule in the following?
Text Solution
|
- Which of the follownig will show geometrical isomerism?
Text Solution
|
- Correct IUPAC name of given compound is
Text Solution
|
- Which of the following pair is correct representation of resonating st...
Text Solution
|
- Which of option is incorrect?
Text Solution
|
- Which of the following types of solids is most likely to be soft, have...
Text Solution
|
- For reaction A(aq.)to2B(aq.), if 50% reaction occurs in 10 minutes and...
Text Solution
|
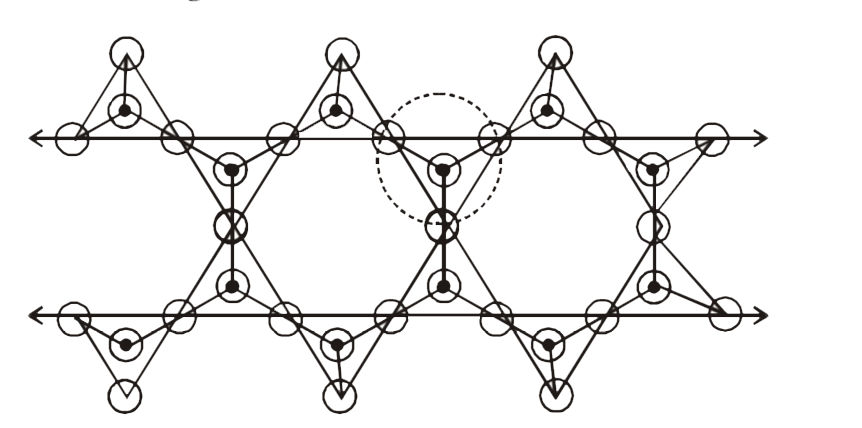
 encircled tetrahedral unie `=3`
encircled tetrahedral unie `=3`