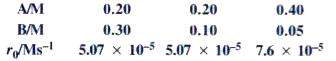Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
ICSE|Exercise PROBLEM |165 VideosCHEMICAL KINETICS
ICSE|Exercise INTEXT QUESRTIONS|81 VideosBIOMOLECULES
ICSE|Exercise ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)|21 VideosCHEMISTRY IN EVERYDAY LIFE
ICSE|Exercise EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))|19 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL KINETICS-ISC EXAMINATION QUESTIONS (NUMERICAL PROBLEMS )
- In a reaction between A and B, the initial rate of reaction was measur...
Text Solution
|
- A first order reaction is 50% complete in 30 minutes at 27^@C. Calcula...
Text Solution
|
- The initial rate of a reaction A+B to Products is doubled when the co...
Text Solution
|
- Consider the reaction, A + B to C + D. The initial rate for differ...
Text Solution
|
- Consider the reaction, A + B to C + D. The initial rate for differ...
Text Solution
|
- Consider the reaction, A + B to C + D. The initial rate for differ...
Text Solution
|
- Consider the reaction, A + B to C + D. The initial rate for differ...
Text Solution
|
- Consider the reaction, A + B to C + D. The initial rate for differ...
Text Solution
|
- The rate constant of a first order reaction is 4.5 xx 10^(-2) sec^(-1)...
Text Solution
|
- 1g of strontium - 90 was reduced to 0.953 g after two years. Calculate...
Text Solution
|
- Show that the time required for the completion of 75% of a reaction of...
Text Solution
|
- A study of chemical kinetics of the reaction, A+B to Products, gave t...
Text Solution
|
- A study of chemical kinetics of the reaction, A+B to Products, gave t...
Text Solution
|
- A study of chemical kinetics of the reaction, A+B to Products, gave t...
Text Solution
|
- In a first order reaction, 10% of the reactant is consumed in 25 minut...
Text Solution
|
- In a first order reaction, 10% of the reactant is consumed in 25 minut...
Text Solution
|
- In a first order reaction, 10% of the reactant is consumed in 25 minut...
Text Solution
|
- If the half-life period for a first order reaction is 69.3 seconds, wh...
Text Solution
|
- The slope of the line in the graph of log k (k = rate constant) versus...
Text Solution
|
- A substance decomposes by following first order kinetics. If 50% of th...
Text Solution
|
- A first order reaction is 50% complete in 30 minutes at 27^@C. Calcula...
Text Solution
|