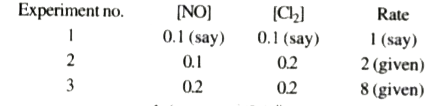Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
ICSE|Exercise INTEXT QUESRTIONS|81 VideosCHEMICAL KINETICS
ICSE|Exercise EXERCISE (PART- I (OBJECTIVE QUESRTIONS)A.FILL IN THE BLANKS) |54 VideosCHEMICAL KINETICS
ICSE|Exercise ISC EXAMINATION QUESTIONS (NUMERICAL PROBLEMS )|57 VideosBIOMOLECULES
ICSE|Exercise ISC EXAMINATION QUSTIONS (PART-I)(DESCRIPTIVE QUESTIONS)|21 VideosCHEMISTRY IN EVERYDAY LIFE
ICSE|Exercise EXERCISE (PART - II (DESCRIPTIVE QUESTIONS) (LONG ANSWER QUESTIONS))|19 Videos
Similar Questions
Explore conceptually related problems
ICSE-CHEMICAL KINETICS-PROBLEM
- Show that in case of a first order reaction, the time required for 99....
Text Solution
|
- The following rate data were obtained at 300 K for the reaction 2A ...
Text Solution
|
- The rate of reaction, 2NO + Cl2 to 2NOCI is doubled when concentrati...
Text Solution
|
- For the following reaction, 2H2 +2NO hArr 2H2 O +N2 the follow...
Text Solution
|
- The initial concentration of N2 O5 in the following first order reacti...
Text Solution
|
- For the thermal decomposition of azomethane, CH3 N2 CH3 at 600 K to N...
Text Solution
|
- The following data were obtained during the first order thermal decomp...
Text Solution
|
- Hydrolysis of methyl acetate in aqueous solution has been studied by t...
Text Solution
|
- The kinetics of hydrolysis of methyl acetate in excess of hydrochloric...
Text Solution
|
- While studying the kinetics of the reaction involving conversion of am...
Text Solution
|
- For the reaction A to B +C, the following data were obtained fi...
Text Solution
|
- The optical rotation of sucrose in 0.90 N HCl at various times is give...
Text Solution
|
- The half-life period of a substance is 50 minutes at a certain concent...
Text Solution
|
- At a certain temperature, the half-life period for the decomposition f...
Text Solution
|
- Following data were obtained for the catalytic decomposition of ammoni...
Text Solution
|
- The rate constants of a reaction at 500 K and 700 K are 0.02 s^(-1) an...
Text Solution
|
- The first order rate constant for the decomposition of ethyl iodide by...
Text Solution
|
- The value of rate constant for a second order reaction is 6.7 xx 10^(-...
Text Solution
|
- Rate constant k of a reaction varies with temperature according to equ...
Text Solution
|
- If a first order reaction has activation energy of 25000 cal and a fre...
Text Solution
|