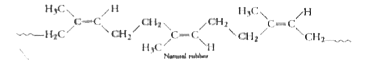
Text Solution
Verified by Experts
Topper's Solved these Questions
POLYMERS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE ( LONG ANSWER TYPE QUESTIONS)|3 VideosPOLYMERS
ARIHANT PUBLICATION|Exercise ODISHA BUREAU.S TEXTBOOK SOLUTIONS (A. MULTIPLE CHOICE TYPE QUESTIONS )|16 VideosPOLYMERS
ARIHANT PUBLICATION|Exercise QUESTIONS FOR PRACTICE ( SHORT ANSWER TYPE I QUESTIONS)|15 VideosPHENOLS
ARIHANT PUBLICATION|Exercise CHAPTER PRACTICE (Long Answer Type Questions )|1 VideosQUESTION PAPER 2019
ARIHANT PUBLICATION|Exercise GROUP C|11 Videos
Similar Questions
Explore conceptually related problems
ARIHANT PUBLICATION-POLYMERS-QUESTIONS FOR PRACTICE ( SHORT ANSWER TYPE II QUESTIONS)
- How are polymers classified on the basis of their structure ?
Text Solution
|
- Distinguish between the terms homopolymer and copolymer. Give one exam...
Text Solution
|
- How can you differentiate between addition and condensation polymerisa...
Text Solution
|
- Differentiate between rubbers and plastics on the basis of intermolecu...
Text Solution
|
- Write the free radical mechanism for the polymerisation of ethene.
Text Solution
|
- Write the names of monomers of the following polymers:
Text Solution
|
- How is dacron obtained from ethylene glycol and terephthalic acid?
Text Solution
|
- How does the presence of double bonds in rubber molecules influence th...
Text Solution
|
- What is the purpose of vulcanisation of rubber?
Text Solution
|
- Write the names and structures of the monomers of the following polyme...
Text Solution
|
- Write the names and structures of the monomers of the following polyme...
Text Solution
|
- Write the names and structures of the monomers of the following polyme...
Text Solution
|
- Write the names and structures of the monomers of the following polyme...
Text Solution
|
- Give an example of polyester used as a synthetic fibre.
Text Solution
|
- Name the compounds from which polyester is prepared.
Text Solution
|
- What type of polymérisation takes place during the formation of the po...
Text Solution
|