A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
QUESTION PAPER 2020
KVPY PREVIOUS YEAR|Exercise PART-I (CHEMISTRY )|20 VideosQUESTION PAPER 2020
KVPY PREVIOUS YEAR|Exercise PART-II(CHEMISTRY)|10 VideosQUESTION PAPER 2020
KVPY PREVIOUS YEAR|Exercise PART-II(CHEMISTRY)|10 VideosQUESTION PAPER 2013
KVPY PREVIOUS YEAR|Exercise PART-II ( CHEMISTRY)|10 VideosSOLVED PAPER 2018
KVPY PREVIOUS YEAR|Exercise EXAMPLE|30 Videos
Similar Questions
Explore conceptually related problems
KVPY PREVIOUS YEAR-QUESTION PAPER 2020-PART-II : CHEMISTRY
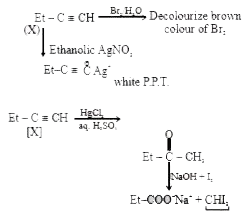
- A hydrocarbon X with molecular fomula C4 H6 decolorizes bromine water ...
Text Solution
|
- 0.102 g of an organic compound X was oxidized with fuming nitric acid....
Text Solution
|
- The specific heat of a certain substance is 0.86 J g^(-1) K^(-1) . Ass...
Text Solution
|
- Strength of a H2 O2 solution is labelled as 1.79 N. its strength can a...
Text Solution
|
- The isotherms of a gas are shown below : Among the following ...
Text Solution
|