Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .6 Colligative Properties And Determination of Molar Mass)|28 VideosSOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .7 Abnormal Molar Mass)|13 VideosSOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .4 Vapour Pressure of Liquid Solutions)|9 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 VideosSTRUCTURE OF ATOM
BETTER CHOICE PUBLICATION|Exercise NUMERICAL PROBLEMS |6 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-SOLUTIONS-Question Bank (2 .5 Ideal and non - ideal solutions)
- Mention three characteristics of ideal solutions . What cause deviatio...
Text Solution
|
- Define Ideal and non-ideal solution. Give examples each of ideal and n...
Text Solution
|
- Explain different types of non-ideal solutions. What is the cause of ...
Text Solution
|
- Write differences between ideal and non-ideal solutions.
Text Solution
|
- What type of deviation is shown by a mixture of benzene and ethyl al...
Text Solution
|
- What type of deviations is shown by a mixture of ethyl alcohol and cy...
Text Solution
|
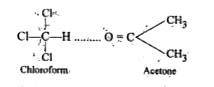
- What type of deviation is shown by a mixture of acetone and chlorofor...
Text Solution
|
- Write two differences between solution and emulsion.
Text Solution
|
- What are Azeotropes ?
Text Solution
|
- Define Azeotropic mixture. Give one example.
Text Solution
|
- What are the differences between minimum boiling azeotropes and maxim...
Text Solution
|