Text Solution
Verified by Experts
Topper's Solved these Questions
SOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .7 Abnormal Molar Mass)|13 VideosSOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Numerical Problems (PSEB 2008)|6 VideosSOLUTIONS
BETTER CHOICE PUBLICATION|Exercise Question Bank (2 .5 Ideal and non - ideal solutions)|11 VideosPOLYMERS
BETTER CHOICE PUBLICATION|Exercise QUESTIONS |42 VideosSTRUCTURE OF ATOM
BETTER CHOICE PUBLICATION|Exercise NUMERICAL PROBLEMS |6 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-SOLUTIONS-Question Bank (2 .6 Colligative Properties And Determination of Molar Mass)
- Define molar elevation constant.
Text Solution
|
- How will you show that elevation in boiling point is a colligative pro...
Text Solution
|
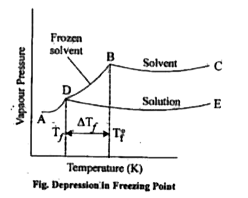
- Explain depression in freezing point. How depression in freezing poi...
Text Solution
|
- Define molar depression constant
Text Solution
|
- How will you show that depression in freezing point is a colligative p...
Text Solution
|
- How will you show that depression in freezing point is a colligative p...
Text Solution
|
- The boiling point increases and freezing point decreases when sodium ...
Text Solution
|
- Sodium chloride solution freezes at lower temparature than water but b...
Text Solution
|
- Out of 1 M urea and 1M KCl solution, which has higher freezing point?
Text Solution
|
- What are antifreeze solutions ?
Text Solution
|
- In cold concentries, a solution of water and ethylene glycol is use...
Text Solution
|
- Why is it advised to add ethylene glycol to water in a car radiator wh...
Text Solution
|
- What is de-icing agent ? How does it function ?
Text Solution
|
- Define osmosis. What is the difference between osmosis and diffusion...
Text Solution
|
- What is Osmotic pressure?
Text Solution
|
- Why determination of osmotic pressure is preffered for finding molecul...
Text Solution
|
- Show that osmotic pressure is a colligative property?
Text Solution
|
- What is a semi-permeable membrane ?
Text Solution
|
- What are isotonic,hypertonic and hypotonic solutions.
Text Solution
|
- Derive the relationship to find the condition for isotonic solutions.
Text Solution
|