Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise NERNST EQUATION|4 VideosELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise CONDUCTANCE OF ELECTROLYTIC SOLUTIONS|21 VideosELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise Numerical Problems|69 VideosCO-ORDINATION COMPOUNDS
BETTER CHOICE PUBLICATION|Exercise QUESTION FROM PREVIOUS BOARD EXAMINATION|59 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
BETTER CHOICE PUBLICATION|Exercise Question Bank (6.6 OXIDATION-REDUCTION)|15 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ELECTROCHEMISTRY-GALVANIC CELLS
- What do you understand by standard reduction potential of electrode?
Text Solution
|
- Give the construction and working of Zn/CuSO(4) or Daniel cell.
Text Solution
|
- In a galvanic cell,
Text Solution
|
- What do you understand by normal hydrogen electrode ? Give its structu...
Text Solution
|
- What is salt bridge? give its functions.
Text Solution
|
- Which type of reaction occurs at cathode in a galanic cell ?
Text Solution
|
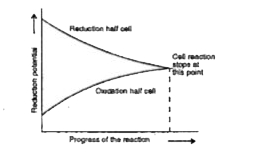
- Why electrochemical cell stops working after sometimes ?
Text Solution
|
- Distinguish between emf and potential difference.
Text Solution
|
- Name the factors on which electrode potential depends?
Text Solution
|
- Why is it not possible to measure the single electrode potential?
Text Solution
|
- What flows in the internal circuit of a galvanic cell ?
Text Solution
|
- What is e.m.f. of the cell, when the cell reaction attains equilibrium...
Text Solution
|
- Can a Galvanic cell work without a salt bridge?
Text Solution
|
- Can you store copper sulphate solutions in a zinc pot?
Text Solution
|
- An aqueous solution containing 1 M each of Au^(3+),Cu^(2+),Ag^+,Li^+ i...
Text Solution
|
- Can we store copper sulphate solution in iron vessel? Give suitable ex...
Text Solution
|
- Can a nickel spoon be used to stir a solution of copper sulphate ? Sup...
Text Solution
|
- What is electrochemical series? How it used to determine the e.m.f. of...
Text Solution
|