Text Solution
Verified by Experts
Topper's Solved these Questions
ELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise FUEL CELLS|8 VideosELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise Numerical Problems|69 VideosELECTROCHEMISTRY
BETTER CHOICE PUBLICATION|Exercise CONDUCTANCE OF ELECTROLYTIC SOLUTIONS|21 VideosCO-ORDINATION COMPOUNDS
BETTER CHOICE PUBLICATION|Exercise QUESTION FROM PREVIOUS BOARD EXAMINATION|59 VideosGENERAL PRINCIPLES AND PROCESS OF ISOLATION OF ELEMENTS
BETTER CHOICE PUBLICATION|Exercise Question Bank (6.6 OXIDATION-REDUCTION)|15 Videos
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-ELECTROCHEMISTRY-ELECTROLYTIC CELLS AND ELECTROLYSIS
- Which allotrope of carbon is used in making electrodes ?
Text Solution
|
- What are inert electrodes ? Give two examples.
Text Solution
|
- Define electrochemical equivalent of substance.
Text Solution
|
- How much amount of substance is deposited by passing one Faraday of el...
Text Solution
|
- what is electrolysis?
Text Solution
|
- Predict the products of electrolysis in each of the following: An aque...
Text Solution
|
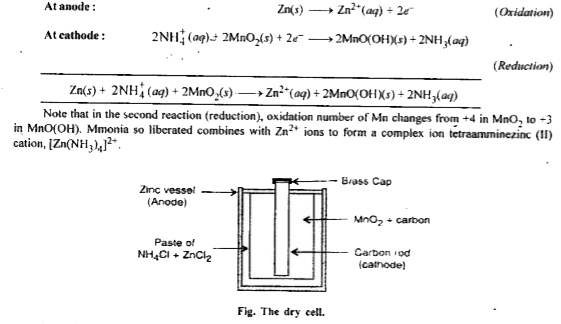
- Write short note on dry cell.
Text Solution
|
- Write short note on lead storage battery.
Text Solution
|
- Write two difference between primary cells and secondary cells.
Text Solution
|
- Describe Ni-Cd storage cell.
Text Solution
|