Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Explain Ostwald process for manufacturing nitric acid. Draw structure ...
Text Solution
|
- Illustrate how copper metal can give different products on reaction wi...
Text Solution
|
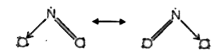
- Why does NO2 dimerise ?
Text Solution
|
- What is the covalence of nitrogen in N2O5 ?
Text Solution
|
- Why does nitric oxide become broen when released inn air?
Text Solution
|
- Ionic solids conduct electricity in the molten state but not in the so...
Text Solution
|
- Which gas is evolved when concentrated HCl is added to powdered potass...
Text Solution
|
- Explain the difference in the structures of white and red phosphorus.
Text Solution
|
- Draw the structure of P4O10.
Text Solution
|
- What happens when white phosphorus is heated with concentrated NaOH so...
Text Solution
|
- Explain why orthophosphorus acid, H3 PO3 is diprotic .
Text Solution
|
- How do you account for the reducing behaviour of H3P02 on the basis of...
Text Solution
|
- Why the basicity of all the acids of phosphorus is different?
Text Solution
|
- Give the structure and basicity of H3PO4.
Text Solution
|
- All the five bonds in PCl5 are not equivalent justify.
Text Solution
|
- Draw the structure of PCl3.
Text Solution
|
- Why are pentahalides more covalent than trihalides ?
Text Solution
|
- Why does NO2 dimerise ?
Text Solution
|
- Write two reactions of HNO3 with non-metals to show the oxidising char...
Text Solution
|
- Explain the acidic character of Perchloric acid compared to sulphuric ...
Text Solution
|