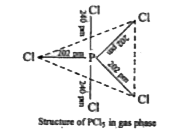
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Why the basicity of all the acids of phosphorus is different?
Text Solution
|
- Give the structure and basicity of H3PO4.
Text Solution
|
- All the five bonds in PCl5 are not equivalent justify.
Text Solution
|
- Draw the structure of PCl3.
Text Solution
|
- Why are pentahalides more covalent than trihalides ?
Text Solution
|
- Why does NO2 dimerise ?
Text Solution
|
- Write two reactions of HNO3 with non-metals to show the oxidising char...
Text Solution
|
- Explain the acidic character of Perchloric acid compared to sulphuric ...
Text Solution
|
- Why white phosphorus is more reactive than red phosphorus?
Text Solution
|
- What happens when ammonium nitrate is heated ?
Text Solution
|
- What is laughing gas ?
Text Solution
|
- Account for the following: The +2 oxidation state of lead is more stab...
Text Solution
|
- Inert pair effect strongly exists in heavy p-block elements. Comment.
Text Solution
|
- Why does R3 P=O exist but R3 N =O does not (R-alkyl group) ?
Text Solution
|
- write the chemical equatikons when Ammonia reacts with aqueous FeCl3.
Text Solution
|
- Write the chemical equations when Ammonia reacts with aqueous AlCl3.
Text Solution
|
- Write the chemical equations when Ammonia reacts with aqueous CrCl3.
Text Solution
|
- Write the chemical equations when Zinc reacts with dilute HNO3.
Text Solution
|
- Write the chemical equations when Zinc reacts with dilute HNO3.
Text Solution
|
- Write the chemical equations when: Zinc reacts with cone. HNO3
Text Solution
|