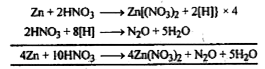Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Write the chemical equations when Ammonia reacts with aqueous CrCl3.
Text Solution
|
- Write the chemical equations when Zinc reacts with dilute HNO3.
Text Solution
|
- Write the chemical equations when Zinc reacts with dilute HNO3.
Text Solution
|
- Write the chemical equations when: Zinc reacts with cone. HNO3
Text Solution
|
- PCl5 is known but PI5 is not known. Why?
Text Solution
|
- NCl3 is readily hydrolysed while NF3 does not. Explain.
Text Solution
|
- NH3 is a strong base but NF3 does not show any basic property. Why?
Text Solution
|
- Why nitric acid acts on oxidising agent ? How it oxdises Sulphur to su...
Text Solution
|
- Why nitric acid acts as an oxididing agent? How it oxidises: Ferrous S...
Text Solution
|
- Discuss abnormal behaviour of oxygen.
Text Solution
|
- Why water is a liquid and hydrogen sulphide is a gas?
Text Solution
|
- Sulphur show +4 and +6 oxidation stae in their compounds but oxygen ca...
Text Solution
|
- Why SF6 is known but OF6 is not known
Text Solution
|
- Explain why H2O is a liquid but H2S is a gas at room temperature.
Text Solution
|
- How aerosols are depleting ozone layer?
Text Solution
|
- Why is H2S less acidic than H2 Te ?
Text Solution
|
- Why H 2 S is acidic wliile H2 O is nentral ?
Text Solution
|
- Why is H2S less acidic than H2 Te ?
Text Solution
|
- What is the oxidation state of phosphorus in H3PO3 , Ca3P2 , Na3PO4 an...
Text Solution
|
- Sulphur exhibits greater tendency for catenation than seleniun. Explai...
Text Solution
|