Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Sulphur show +4 and +6 oxidation stae in their compounds but oxygen ca...
Text Solution
|
- Why SF6 is known but OF6 is not known
Text Solution
|
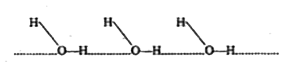
- Explain why H2O is a liquid but H2S is a gas at room temperature.
Text Solution
|
- How aerosols are depleting ozone layer?
Text Solution
|
- Why is H2S less acidic than H2 Te ?
Text Solution
|
- Why H 2 S is acidic wliile H2 O is nentral ?
Text Solution
|
- Why is H2S less acidic than H2 Te ?
Text Solution
|
- What is the oxidation state of phosphorus in H3PO3 , Ca3P2 , Na3PO4 an...
Text Solution
|
- Sulphur exhibits greater tendency for catenation than seleniun. Explai...
Text Solution
|
- Elements of Group 16 generally show lower value of first ionisation en...
Text Solution
|
- Oxygen gas is inert at room temperature why?
Text Solution
|
- Give two methods of preparation of dioxygen in laboratory and give its...
Text Solution
|
- Discuss the preparation of ozone by Sieman's Ozoniser.
Text Solution
|
- Write two uses of ozone.
Text Solution
|
- Why does O3 act as a powerful oxidising agent ?
Text Solution
|
- What is the role of SO2 in air pollution?
Text Solution
|
- How will ozone oxidise the following : Potassium nitrite to potassium ...
Text Solution
|
- What will be the oxidation number of S in SO2Cl2 and of P in Ca3P2?
Text Solution
|
- How will ozone oxidise the following : Potassium manganate to potassiu...
Text Solution
|
- How does fluorine and chlorine react with water. Only write chemical r...
Text Solution
|