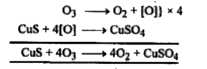
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- What is the role of SO2 in air pollution?
Text Solution
|
- How will ozone oxidise the following : Potassium nitrite to potassium ...
Text Solution
|
- What will be the oxidation number of S in SO2Cl2 and of P in Ca3P2?
Text Solution
|
- How will ozone oxidise the following : Potassium manganate to potassiu...
Text Solution
|
- How does fluorine and chlorine react with water. Only write chemical r...
Text Solution
|
- How does O3 oxidises the Acidified ferrous sulphate to fertic sulphate...
Text Solution
|
- What is tailing of mercury ?
Text Solution
|
- How ozone reacts with mercury.
Text Solution
|
- Discuss the shape of SF6
Text Solution
|
- Discuss the shape of SF4?
Text Solution
|
- SF6 is known but SCl6 is not known. Explain.
Text Solution
|
- Sulphur hexafluoride is used as a gaseous electrical insulator. Explai...
Text Solution
|
- SF4 is easily hydrolysed whereas SF6 is not easily hydrolysed. Why ?
Text Solution
|
- Why SF6 in inert towards hydrolysis ?
Text Solution
|
- Why is SF6 much less reactive than SF4 ?
Text Solution
|
- Why SF6 is known but SH6 is not known ?
Text Solution
|
- Which form of sulphur shows paramagnetic behaviour and why ?
Text Solution
|
- Why does sulphur in vapour state exhibit paramagnetic character ?
Text Solution
|
- Explain that SO2 can act as an oxidising agent as well as a reducing a...
Text Solution
|
- Explain that SO2 can act as an oxidising agent as well as a reducing a...
Text Solution
|