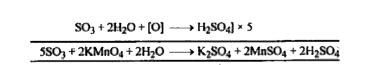
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Write the reaction of SO2 with Cl2
Text Solution
|
- Write the reaction of SO2 with Cl2
Text Solution
|
- Give the oxidation of Na2SO3 by acidified KMnO4.
Text Solution
|
- Give the formula and structures of different oxo-acids of sulphur ?
Text Solution
|
- Describe the manufacture of H2SO4 by contact process?
Text Solution
|
- Explain the manufacture of sulphuric acid by contact process.
Text Solution
|
- Write the conditions for maximum yield of H2SO4 by contact process.
Text Solution
|
- Give an example of a reaction in which H2 SO4 behaves as a strong acid...
Text Solution
|
- Give an example of a reaction in which H2 SO4 behaves as a dehydrating...
Text Solution
|
- Give an example of a reaction in which H2 SO4 behaves as an oxidising ...
Text Solution
|
- Describe the commerical uses of sulphuric acid.
Text Solution
|
- Mention three areas in which H2SO4 plays an important role.
Text Solution
|
- Why conc. H2 SO4 is viscous and has high boiling point ?
Text Solution
|
- Why is K(a2) << k(a1) for H2SO4 in water?
Text Solution
|
- Why conc. sulphuric acid is always diluted by adding sulphuric acid to...
Text Solution
|
- Write the chemical equation when: Oxalic and reacts with concentrated ...
Text Solution
|
- Write the chemical equation when: Formic acid reacts with concentrated...
Text Solution
|
- Write the chemical equations when: Sugar reacts with conc. H2SO4.
Text Solution
|
- SO3 has zero dipole moment. Why ?
Text Solution
|
- Why does fluoriue show anomalous behaviour in its group ?
Text Solution
|