Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Write the chemical equation when: Formic acid reacts with concentrated...
Text Solution
|
- Write the chemical equations when: Sugar reacts with conc. H2SO4.
Text Solution
|
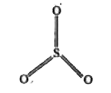
- SO3 has zero dipole moment. Why ?
Text Solution
|
- Why does fluoriue show anomalous behaviour in its group ?
Text Solution
|
- Why are halogens most reactive ?
Text Solution
|
- Explain why Bond dissociation energy of F2 is less than that of Cl2 ?
Text Solution
|
- Explain why F-F bond in fluorine is weaker than Cl-Cl bond in chlori...
Text Solution
|
- Explain the following : HF is a weaker acid than HI.
Text Solution
|
- Explain the following : Iodine is more soluble in KI solution than i...
Text Solution
|
- Explain the following : Fluorine does not show positive oxidation st...
Text Solution
|
- Explain the following : Iodine forms I3^- but F2^ does not form F3^-...
Text Solution
|
- Explain: Electron gain enthalpy of chlorine is more negative than fluo...
Text Solution
|
- Why electron affinity of fluorine is less than that of chlorine ?
Text Solution
|
- Boiling point of HCI is lower than HF . Explain why?
Text Solution
|
- Why are halogens coloured ?
Text Solution
|
- Halogens have maximum negative electron gain enthalpy in the respectiv...
Text Solution
|
- Electron gain enthalpy of chlorine is more negative as compared to flu...
Text Solution
|
- Considering the parameters such as bond dissociation enthalpy, electro...
Text Solution
|
- Why is fluorine a very reactive halogen ?
Text Solution
|
- Account for the following: Among the halogens F2 is the strongest oxid...
Text Solution
|