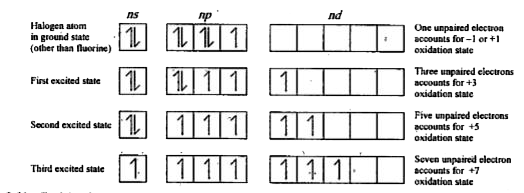
Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- Why is fluorine a very reactive halogen ?
Text Solution
|
- Account for the following: Among the halogens F2 is the strongest oxid...
Text Solution
|
- Fluorine exhibits only - 1 oxidation state whereas other halogens exhi...
Text Solution
|
- Explain why Most metal fluoride are ionic in nature than metal chlo...
Text Solution
|
- Explain why Hydrogen fluoride is a weaker acid than hydrogen chlorid...
Text Solution
|
- Why are halogens strong oxidising agents?
Text Solution
|
- Explain why inspite of nearly the same electronegativity, nitrogen for...
Text Solution
|
- Write the reactions of F2 and Cl2 with water.
Text Solution
|
- Arrange the following in the order of property indicated for each set:...
Text Solution
|
- Arrange the following in the order of property indicated for each set:...
Text Solution
|
- Write the balanced chemical equation for the reaction of CI2 with hot ...
Text Solution
|
- Write the balanced chemical equation for the reaction of CI2 with hot ...
Text Solution
|
- Write the balanced chemical equation for the reaction of CI2 with hot ...
Text Solution
|
- Anita coloured 3/8 of the circle with yellow colour and 4/8 of the cir...
Text Solution
|
- Name two poisonous gases which can be prepared from chlorine gas.
Text Solution
|
- Give the reason for bleaching action of Cl2.
Text Solution
|
- Bleaching of flowers by chlorine is permanent while that by sulphur di...
Text Solution
|
- How can you prepare Cl2 from HCl and HCl from Cl2? Write reactions onl...
Text Solution
|
- Write balanced equations for the following: NaCl is heated with sulphu...
Text Solution
|
- Write balanced equations for the following: Chlorine gas is passed int...
Text Solution
|