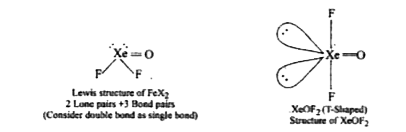Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-THE P-BLOCK ELEMENTS-QUESTION BANK
- What inspired N. Bartlett,for carrying out the reaction between Xe and...
Text Solution
|
- Among noble gases, only Xe is known to form chemical compounds. Why ?
Text Solution
|
- Why do noble gases form compounds with fluorine and oxygen ?
Text Solution
|
- How are Xenon fluorides XeF2, XeF4 and XeF6 prepared ?
Text Solution
|
- Discuss the structure of the XeF4 on the basis of VSEPR theory.
Text Solution
|
- Discuss the structure of XeF2 on the basis of VSEPR teory.
Text Solution
|
- How are XeO3 and XeOF4 prepared?
Text Solution
|
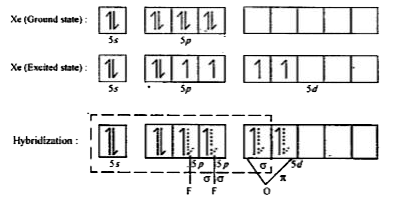
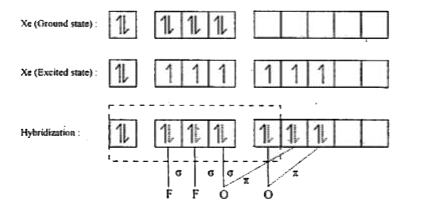
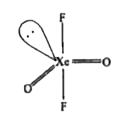
- Discuss the shapes of XeO3, XeOF4, XeOF2, and XeO2 F2
Text Solution
|
- Discuss the hydrolysis of XeF3. Does the hydrolysis of XeF6 lead to a ...
Text Solution
|
- Give equation for the following: XeF2 + H2 O to
Text Solution
|
- Give equation for the following: XeF6 + H2 O
Text Solution
|
- Give equation for the following: XeF2 + PF5 to
Text Solution
|
- Give equation for the following: XeF6 + NaF to
Text Solution
|
- Give the structure of XeOF2 and state of hybridization of Xe in it.
Text Solution
|
- Why is helium used in diving apparatus?
Text Solution
|
- List the uses of neon and argon gases.
Text Solution
|
- Give uses of argon, helium, neon, xenon and krypton gases.
Text Solution
|
- Give the formula and describe the structure of a noble gas species whi...
Text Solution
|
- Give the formula of the noble gas species which is isostructural with ...
Text Solution
|
- Give the formula of the noble gas species which is isostructural with ...
Text Solution
|