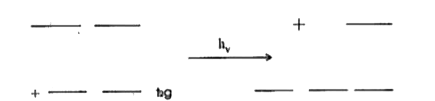Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
BETTER CHOICE PUBLICATION-CO-ORDINATION COMPOUNDS-QUESTION FROM PREVIOUS BOARD EXAMINATION
- Discuss structure of [Co(NH3)6]^(3+) ion the basis of V.B.T.
Text Solution
|
- What is the state of hybridisation and geometry in [Ni(CN)4]^(-2)
Text Solution
|
- What is the state of hybridisation and geometry in [Ni(CN)4]^(-2)
Text Solution
|
- Explain [Fe(H2O)6]^(3+) is paramagnetic.
Text Solution
|
- With the help of crystal field theory, predict the number of unpaired ...
Text Solution
|
- predict the number of unpaired electrons in [CoF6]^(3-) and [Co(NH3)6]...
Text Solution
|
- With the help of the crystal field theory predict the number of unpair...
Text Solution
|
- Account for the different magnetic behaviour of hexacyanoferrate (III)...
Text Solution
|
- Explain : [Co(CN)6]^(3-) is diamagnetic while [CoF6]^(3-) is paramagne...
Text Solution
|
- Draw the geometrical isomers of [Co(en)2CI2]^+ ion.Which of these is o...
Text Solution
|
- Draw the geometrical isomers of [Co(en)2CI2]^+ ion.Which of these is o...
Text Solution
|
- Discuss the main postulates of valence bond theory.
Text Solution
|
- Write the name of ionisation isomer of the compound [Co(NH3)5 Br]SO4.
Text Solution
|
- Draw the geometrical isomers of [Co(en)2CI2]^+ ion.Which of these is o...
Text Solution
|
- Write the name of ionisation isomer of the compound [CO(NH3)4 Cl2]NO2
Text Solution
|
- Draw the geometrical isomers of [Pt(NH3)2.CI2]. Which of these is opti...
Text Solution
|
- Draw the geometrical isomers of [Pt(NH3)2.CI2]. Which of these is opti...
Text Solution
|
- Explain the difference between a weak field ligand and a strong field ...
Text Solution
|
- Write the structure and hybridisation of the central atom in [CoCl2 (N...
Text Solution
|
- [Ti (H2O)6]^(3+) is coloured while [Sc(H2O)6]^(3+) is colourless. Expl...
Text Solution
|