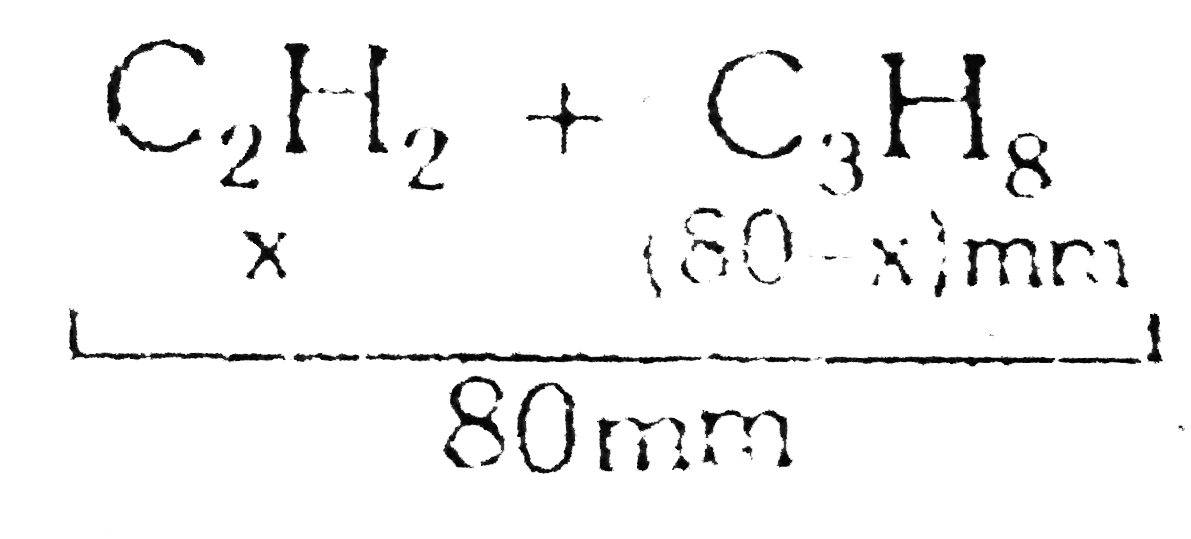A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-MOLE CONCEPT-EXERCISE - 01
- The molarity of the solution containing 2.8% (mass/volume) solution of...
Text Solution
|
- The molality of a sulphuric acid solution is 0.2"mol"//"kg" Calculate ...
Text Solution
|
- What volume of a 0.8M solution contains 100 millimoles of the solute .
Text Solution
|
- 500 mL of a glucose solution contains 6.02 xx 10^(22) molecules. The c...
Text Solution
|
- 50mL of CO is mixed with 20mL of oxygen and sparked. After the reactio...
Text Solution
|
- 14gm of element X combine with 16gm of oxygen. On the basis of this in...
Text Solution
|
- 1.5gm mixture of SiO(2) and Fe(2)O(3) on very strong heating leave a r...
Text Solution
|
- Aspartame an artificial sweetener contains 9.52 "wt". % nitrogen. Ther...
Text Solution
|
- The total number of neutrons present in 10g D(2)O (D "is" 2 H are .
Text Solution
|
- The % of Fe^(+2) in Fe(0.93)O(1.00) is .
Text Solution
|
- The mass precent of oxYgen in 109% oleum is .
Text Solution
|
- A definite amount of gaseous hydrocarbon having ("carbon atoms less t...
Text Solution
|
- C(6)H(5)OH(g)+O(2)rarrCO(2)(g) +H(2)O(l) Magnitude of volume change ...
Text Solution
|
- 10ml of compound containing 'N' and 'O' is mixed with 30ml of H(2) to ...
Text Solution
|
- 200ml of a gaseous mixture containing CO,CO(2) and N(2) on complete co...
Text Solution
|
- When 20ml of mixture of O(2) and O(3) is heated the volume becomes 29m...
Text Solution
|
- A mixture of C(2)H(2) and C(3)H(8) occupied a certain volume at 80mm H...
Text Solution
|
- 20 Ml of a mixture of CO and H(2) were mixed with excess of O(2) and e...
Text Solution
|
- The % by volume of C(4)H(10) in a gaseous mixture of C(4)H(10) and CO ...
Text Solution
|
- An ideal gaseous mixture of ethane (C(2)H(6)) and ethane (C(2)H(4)) oc...
Text Solution
|