Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-ELECTROCHEMISTRY-EXERCISE-04 [B]
- The e.m.f. of cell: H(2)(g) |Buffer| Normal calomal electrode is 0.688...
Text Solution
|
- The direct current of 1.25A was passed through 200mL of 0.25M Fe(@)(SO...
Text Solution
|
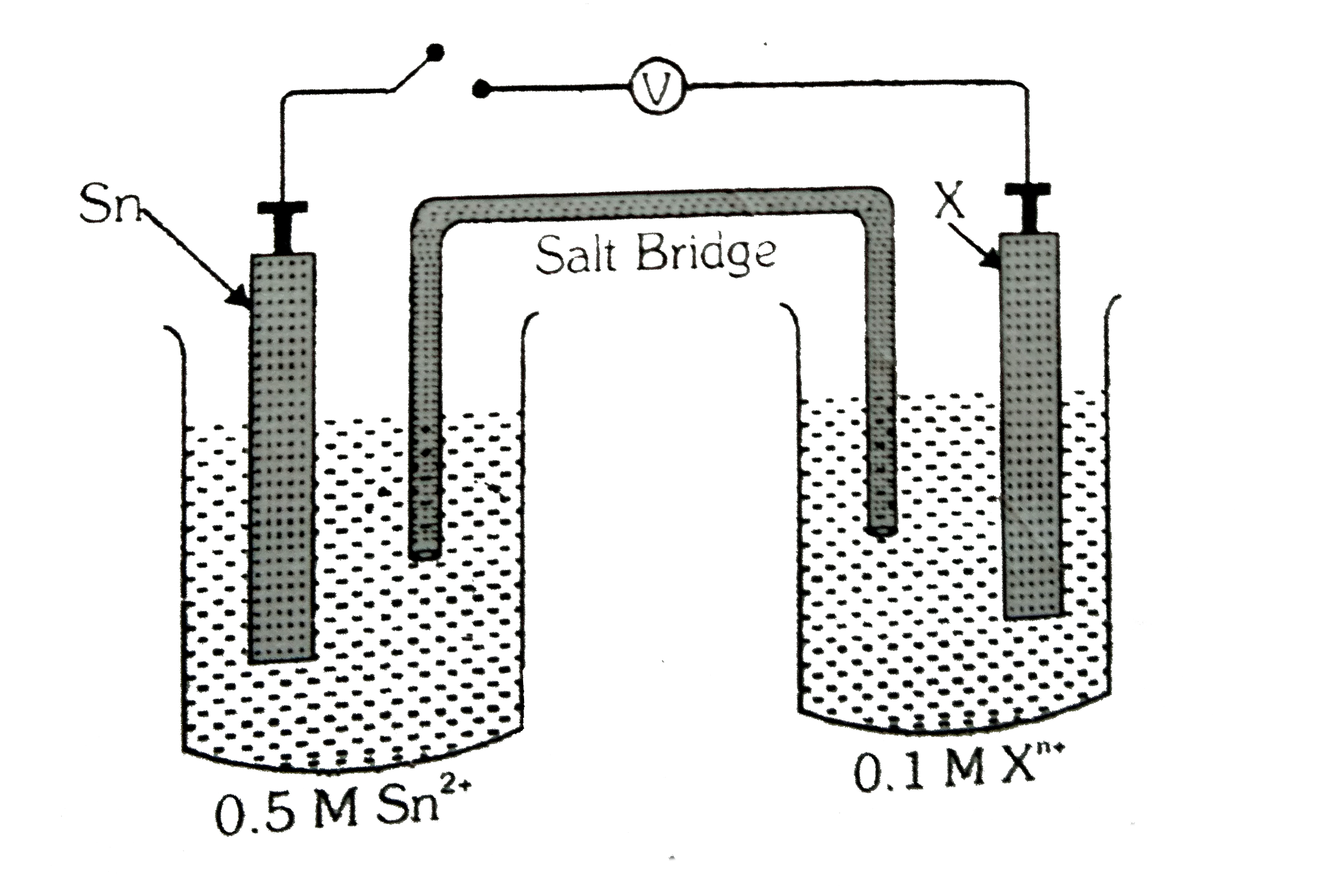
- An electrochemical cell is constructed with an open switch as shown be...
Text Solution
|
- 10g fairly concentrated solution of CuSO(4) is electrolyzed using 0.01...
Text Solution
|
- One of the methods of preparation of per disulphuric acid, H(2)S(2)O(8...
Text Solution
|
- Assume that impure copper contains only iron, silver and a gold as imp...
Text Solution
|
- For the galvanic cell: Ag|AgCI(s)|KCI(0.2M)||KBr(0.001M)|AgBr(s)|Ag, c...
Text Solution
|
- An aqueous solution of NaCl on electrolysis gives H(2)(g), Cl(2)(g), a...
Text Solution
|
- An acidic solution of Cu^(2+) salt containing 0.4g of Cu^(2+) is elect...
Text Solution
|
- In the refining of silver by electrolytic method what will be the weig...
Text Solution
|
- Hydrogen peroxide can be prepared by successive reaction: 2NH(4)HSO(...
Text Solution
|
- Dal lake has water 8.2 xx 10^(12)litre approximately. A power reactor ...
Text Solution
|
- Determine the degree of hydrolysis and hydrolysis constant of aniline ...
Text Solution
|
- The emf of the cell, Pt|H(2)(1atm)|H^(+) (0.1M, 30mL)||Ag^(+) (0.8M)Ag...
Text Solution
|
- A dilute aqueous solution of KCI was placed between two electrodes 10 ...
Text Solution
|
- 100mL CuSO(4) (aq) was electrolyzed using inert electrodes by passing ...
Text Solution
|
- Calculate the equilibrium concentration of all ions in an ideal soluti...
Text Solution
|
- Determine at 298 for cell: Pt|Q, QH(2), H^(+) ||1M KCI |Hg(2)CI(2)|H...
Text Solution
|
- At 25^(@)C, DeltaH(f)(H(2)O,l) =- 56700 J//mol and energy of ionizatio...
Text Solution
|
- Calculate the cell potential of a cell having reaction Ag(2)S +2e^(-) ...
Text Solution
|