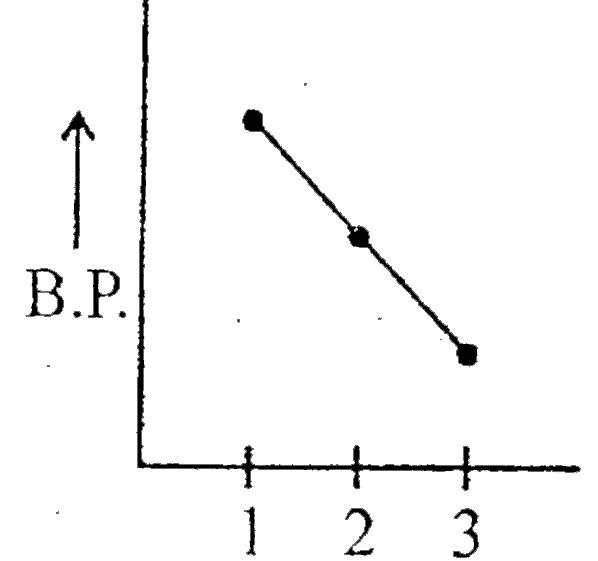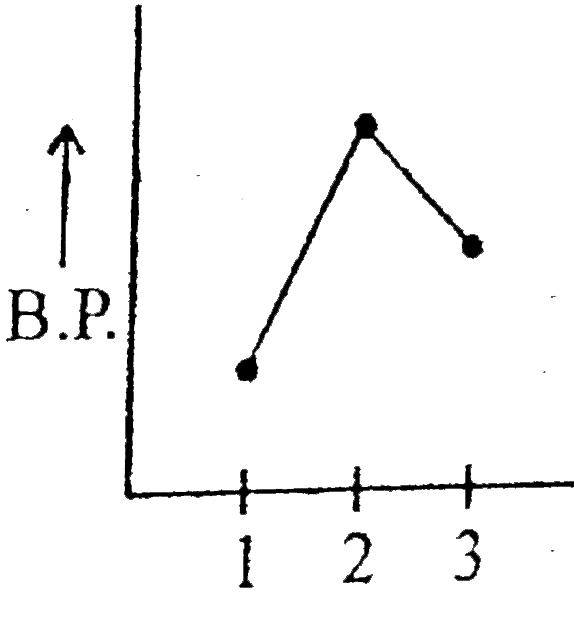A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
ALLEN-TEST PAPERS-PAPER 1
- Which is correct :-
Text Solution
|
- You have given two species :- NOF and NO(2)F and two dipole moments 1....
Text Solution
|
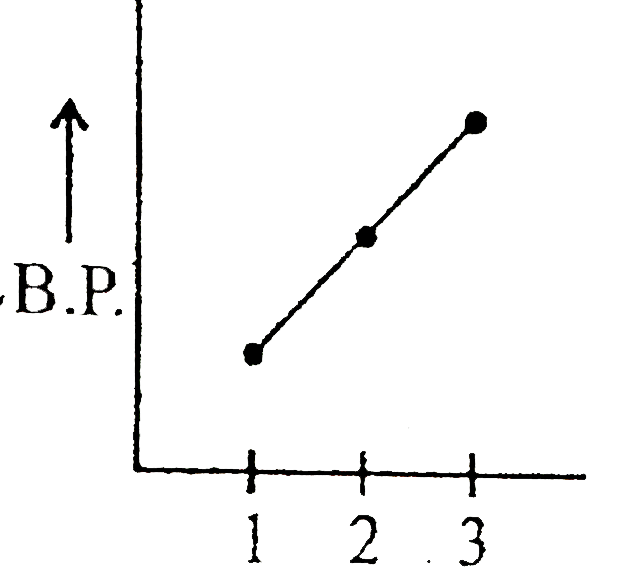
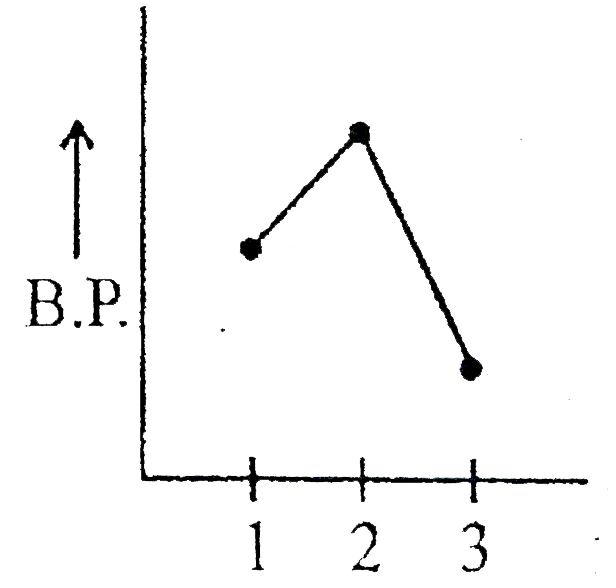
- Which graph represents correct for boiling point of:- (1) Ethane (2) ...
Text Solution
|
- Catenation power order is GeltSiltC then C-C,Si-Si and Ge-Ge bond ener...
Text Solution
|
- The correct order for triple bond energy in CO, N2, CN and C-=C is ,
Text Solution
|
- Which of the following statements is correct:
Text Solution
|
- Then choose the correct statement:-
Text Solution
|
- In the reaction :Cl(2)+OH^(-)rarrCl^(-)+ClO(4)^(-)+H(2)O
Text Solution
|
- Oxidation numbers of Mn in its compounds MnCl(2),Mn(OH)(3),MnO(2) and ...
Text Solution
|
- N(2)+3H(2)hArr2NH(3) if in equilibrium mixture by volume N(2) is 40%, ...
Text Solution
|
- Given the following reaction and equilibrium constant CO(g)+(1)/(2)O...
Text Solution
|
- Approximate p.H,0.1M aqueous H(2)S solution when K(1) and K(2) for H(2...
Text Solution
|
- A buffer solution contains 100mL of 0.01 M CH(3)COOH and 200mL of 0.02...
Text Solution
|
- The heat of combustion of ethylene at 18^(@)C and at constant volume i...
Text Solution
|
- One mole of any substance contains 6.022xx10^23 atoms/molecules. Numbe...
Text Solution
|
- Which of the following statements is correct about the given reaction ...
Text Solution
|
- Two flask A and B of equal capacity contains CO(2) and O(2) gas under ...
Text Solution
|
- The ratio of area covered by second orbit to the first orbit in hydrog...
Text Solution
|
- The wave number of the spectral line in the emission spectrum of hydro...
Text Solution
|
- The shortest wavelength in hydrogen spectrum of Lyman series when R(...
Text Solution
|